MD&M East 2012 Event Coverage
May 16, 2012
Brian Reilly, product director, healthcare materials, at NuSil Technology (Carpinteria, CA) will present "Modification of Silicone Chemistry and Its Influence on Release Rates of Active Pharmaceutical Ingredients (APIs)" at the MD&M East conference program on Monday, May 21. Serving the medical device and other industries, NuSil focuses exclusively on silicone technology and related process development.
MPMN: What features or characteristics of silicone make it desirable for drug-delivery applications?
Reilly: For about 60 years, silicone has been used as a raw material for healthcare applications; it has been used for more than 20 years as a raw material for use in drug-delivery applications and combination products. Silicone is highly chemically stable and biologically inert. In addition, the cured silicone matrix has a high degree of free volume, which facilitates compounding in a variety of soluble APIs. It is this free volume that allows APIs to diffuse through the matrix and be released through the surface of the part or device.
|
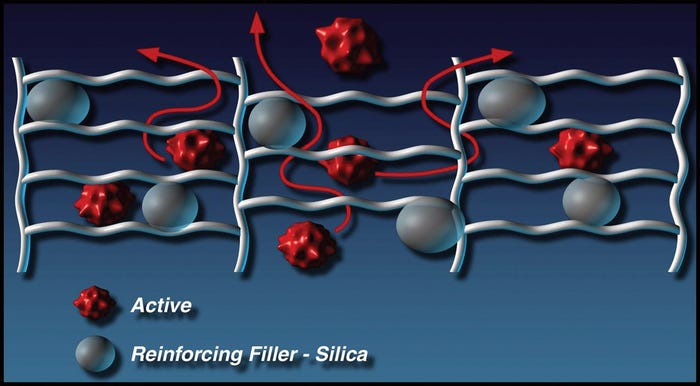
Various obstacles, including crosslink density and reinforcing silica, impact API permeation through a cured silicone matrix. |
Silicones also offer a host of options in terms of physical states and chemistry, such that end-users, in turn, have many options in regards to processing methods, which include extrusion, liquid injection molding, and transfer molding. Silicone formulations can be customized in a variety of ways to facilitate a specific process of fabrication while achieving key mechanical and drug-elution properties as well. Application examples include antimicrobial catheters, contraceptive intravaginal rings (IVRs) and intrauterine devices (IUDs), pacemaker leads with an antiinflammatory active, and transdermal skin patches that range in application from treating high blood pressure to pain management.
MPMN: What factors must medical device companies take into account when planning to incorporate APIs into a silicone system?
Reilly: Initially, medical device companies should determine whether they have the appropriate facilities and quality systems to work with APIs. On a basic level, they should understand the API in question: Is it solid, liquid, or crystalline? How soluble is it in silicone? In combination with loading level, these factors will dramatically influence the types of mixing equipment that will be needed to compound the API into the silicone, as well as the degree of difficulty to do so. Is the API sensitive to light, temperature, or moisture? Will it react with other chemicals? Answers to these questions immediately impact storing and handling of the neat API and heavily affect processing conditions and finished product storage as well.
Regarding temperature stability, many APIs are sensitive to elevated temperatures, while most silicone elastomers require elevated temperatures to cure. This question of temperature compatibility must be answered but, to do so effectively, a customer must also have the analytical capabilities to identify and assay the active and any degradation products the manufacturing and curing processes may cause. In addition to extensive capabilities for identity and purity determination, a medical device company must also have the means to conduct elution testing and content uniformity testing. Lastly, there's inhibition. If a company is hoping to employ a process and design that relies upon the compounding of an API into a two-part platinum-catalyzed silicone, it will first need to determine if there are any incompatibilities between the platinum catalyst and the API. Platinum catalysts are very sensitive to many different elements and chemicals; therefore, it's possible that the API could inhibit or kill the catalyst. In such an instance, other cure chemistries will need to be considered, which could dramatically alter the process design.
MPMN: What challenges does the integration of APIs into a silicone system present for medical device applications?
Reilly: The main challenge is simply stated but often complex to overcome. Applications that depend on a silicone elastomer to function as the platform for the delivery of an API have basic development goals:
o Achieve a method for compounding the API into the silicone that results in good content uniformity without causing degradation of the API
o Achieve a formulation that delivers a cured part with the necessary mechanical properties
o Achieve a formulation that yields a cured part with the specified API release rate
o Achieve a formulation that may be readily processed
What makes these straightforward goals so challenging is that they are often competing with each other. Certain formulary adjustments intended to facilitate a release rate can result in diminished mechanical properties. On the most basic level, the moment the silicone is loaded with an API, its rheology changes--which can often impact processability--and mechanical properties are diminished. Once the product is successfully developed, the next challenge becomes determining the stability of the formulation on the shelf over months and years. Will the API eventually degrade the cure chemistry of the silicone, or will the silicone chemistry slowly degrade the API? Such questions can only be answered with extensive stability studies.
MPMN: How can these issues be overcome or avoided?
Reilly: Work such as this requires true product development, wherein formulations are built, tested, evaluated, modified, built again, etc. To achieve the four goals previously identified, it is often critical to be able to make adjustments to the silicone formulation itself. For example, in the course of our work with drug-delivery product development, NuSil chemists frequently make adjustments to the types and levels of reinforcing media, the types of polymers, and the reactivity thereof. This iterative system of formulation and manufacture requires expertise with silicone chemistry and processes. It also relies heavily upon the ability to quickly and effectively evaluate formulations for processability, mechanical performance, and drug-elution performance. Such evaluation requires a multitude of testing capabilities, including extraction; analytical methods for identity determination and assay; elution testing, intended to model end-use environment and predict performance; and content uniformity testing. Some projects require limited iterations before a successful candidate formulation is achieved, but some require much more--sometimes upwards of 10 iterations. This is why it is critical that a company undertaking this work has or obtains the capacity for quick formulation development and analysis.
You May Also Like