ADHESIVES
Silicone adhesives, along with primers, flame treatment, or plasma treatment, can be used to adhere low-surface-energy plastics to high-strength metals. For this article, several low-surface-energy plastics and high-strength metals were tested with silicone adhesives and primers, as well as with plasma treatment, to achieve cohesive bond failure when performing lap-shear testing. This list of substrates evaluated includes polycarbonate, polyetherimide, polyamide, polyurethane, polymethylmethacrylate, polysulfone, titanium, stainless steel, and aluminum.
Device engineers have many options for joining or sealing parts. One of the common technologies used is bonding or adhesive technology. Because there are so many substrates available, suppliers cannot test each substrate type with every adhesive. However, by testing adhesives on some novel or difficult substrates, inferences can be made that can narrow the choices of adhesives and primers.
Surface Energy and Wetting
Bonding any type of adhesive requires an understanding of surface energy, chemical reactivity, and the mechanisms necessary for a good bond. Surface energy is the thermodynamic effect related to a material's intramolecular forces. This property partially determines how well a liquid wets out, or spreads across, a surface. It is commonly accepted that the substrate's surface energy must exceed that of the adhesive liquid for adequate wetting. Intuitively, the better an adhesive wets out, the more intimate the contact between molecules and, therefore, the more the reactive groups interact or bond. In aggregate, the interactions between the adhesive and substrate make a stronger bond.
|
Table I. (click to enlarge) Table I. Typical surface-energy dyne levels. |
Low-surface-energy materials, such as plastics, do not encourage some adhesives to wet out, which means they are generally poor candidates for bonding. Table I includes some substrates and their surface energies. It is possible to raise the surface energy of some plastics through UV radiation, plasma or corona discharge, or by flame or acid treatments. The presence of oxygen-containing species, such as OH groups, on the surface of a plastic provides reactive sites for silicone adhesive and primer systems, which leads to another key factor: bonding.
Bonding Mechanisms
In addition to adequate surface wetting, a second critical factor to consider is how the adhesive forms a mechanical or chemical bond to the substrate surface. Adhesion can be achieved through a few mechanisms; but, for the purposes of this article, we will focus on mechanical and chemical adhesion.
Mechanical adhesion can best be described as a locking mechanism between an adhesive and a substrate surface. Sanding a substrate surface can produce the topography necessary to form a mechanical bond. These bonds are useful in situations where delamination does not interfere with the device's function.
Chemical adhesion is defined as the chemical bonding of two substrates, either by covalent bonding, hydrogen bonding, or other van der Waals forces. Substrates with reactive groups available for bonding—like OH groups on glass and oxide layers on metals such as aluminum—make this chemical bond easier to achieve. Substrates with inert surfaces, such as graphite and polytetrafluoroethylene (PTFE), can make adhesion difficult.
Substrates
Several substrates were used to test adhesion in the experiment. Here is a list of those materials and discussion of their properties.
|
Figure 1. (click to enlarge) Figure 1. A polycarbonate structure. |
|
Figure 2.(click to enlarge) Figure 2. A polyetherimide structure. |
|
Figure 3.(click to enlarge) Figure 3. A polyamide structure (Nylon 6/6). |
Polycarbonate. Depending on the industry, some substrates are more commonly used than others. The medical device industry represents a large growth area for polycarbonate. Because of its established molding operations, ease of molding, and low weight, device manufacturers often incorporate polycarbonate into devices. The material (see Figure 1) can be found in applications including blood reservoirs, oxygenators, and safety-syringe needle hubs. It is often chosen for its biocompatibility, high-impact strength, and dimensional stability.
The authors used Bayer's Makrolon 2658-1112 polycarbonate. It is FDA-approved for general use and does not have an internal mold-release additive.
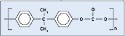
Polyetherimides. Polyetherimides (PEIs) show up in a number of medical device applications, such as medical connectors. GE Plastics' Ultem has become synonymous with the chemical name. Figure 2 shows the chemical structure of polyetherimide.The authors used Ultem 1000 for adhesion testing.
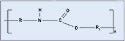
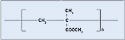
Polyamide. Better known by its trade name, nylon, polyamide is a common plastic with low surface energy, although its surface energy is slightly higher than polycarbonate. It is probably the most diverse thermoplastic and is often used in medical tubing, wire harnesses, catheters, control knobs, and cable ties. Nylon offers great wear, as well as chemical and thermal resistance. The material is also relatively inexpensive. Nylon 6/6, 6, and 12 are the most common types, with the numbers referring to the number of methyl groups occurring on each side of the nitrogen atom (see Figure 3). Dow Vydyne ECO315, Q3211-(RED) was used for these experiments.
|
Figure 4.(click to enlarge) Figure 4. A polyurethane structure. |
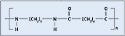
Polyurethane. Catheters, anesthesia masks, and medical tubing all use polyurethane. Its abrasion and chemical resistance make it a popular choice. Polyurethanes can be modified for different durometers, depending on application requirements, but they retain impact strength at low temperatures. Figure 4 depicts the structure of polyurethane. Researchers used Dow Pellethane 2103-55D for these experiments.
|
Figure 5. (click to enlarge) Figure 5. An acrylic structure. |
Polymethylmethacrylate. Polymethylmethacrylate (PMMA), more commonly referred to as acrylic, can be found in various device applications, including blood pumps and filters. Acrylic (see Figure 5) is also referred to as Plexiglas. It is known for its clarity and is often used when resistance to yellowing or embrittlement is critical. For the experiment, researchers used CYRO Industries Cyrolite G20 100.
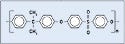
Polysulfones. Their chemical and mechanical stability make polysulfones common for use in housings and reservoirs. The material has thermal-, electrical-, and creep-resistance properties for a range of temperatures (see Figure 6). This material is common in machine components where resistance to high-temperature and electrical properties is important.
|
Figure 6. (click to enlarge) Figure 6. A polysulfone structure. |
|
Figure 7. (click to enlarge) Figure 7. A polyimide film structure (Kapton). |
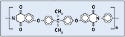
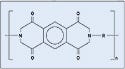
Polyimide. Polyimide is used in catheter tubing and other applications. DuPont's Kapton polyimide film was used for the study (see Figure 7).
Titanium. Titanium is often found in implantable devices such as pacemaker housings and defibrillators, and in guidewires and impeller blades in minimally invasive devices. Because of its favorable strength-to-weight ratio, titanium has become a staple in the orthopedic industry. It also provides excellent corrosion resistance to moisture and many acids and bases. Because of the nature of its protective oxide film, titanium's erosion and cavitation resistance is 20 times greater than that of copper-nickel alloys.
Some applications require the use of an adhesive capable of sealing or bonding metals such as stainless steel, aluminum, or titanium. Titanium and stainless steel are often chosen for their strength, durability, and biocompatibility. Aluminum is often chosen because it can be easily processed for molding, casting, or machining.
Materials
Primers. Primers have become necessary for adhering difficult substrates. Although often used to aid in adhesion, primers add another step to the process. Silane primers are used to promote adhesion between two nonbonding surfaces and are used with silicone adhesives; but they can also be used with other types of adhesives, like epoxies.
Primers usually consist of one or more reactive silanes, a condensation catalyst, and some type of solvent carrier. Reactive silanes typically have two reactive groups: one that is compatible with the substrate and another that is compatible with the adhesive. Some types of groups may be hydrophilic like a silanol (Si-OH) group, or hydrophobic like a 1-octenyl group. These different groups form a compatible interface between incompatible substrates to promote adhesion. Silanes are usually added as moisture-sensitive alkoxy silanes and, in the presence of water and a condensation catalyst, form the priming surface.
The reactive species are typically in concentrations of 5–20% in solvent. The solvent's main purpose is to dilute the surface's reactive species (silanes and condensation catalysts) and to promote a thin film of these species. The silanes hydrolyze in the presence of atmospheric moisture and subsequently react with each other in the presence of a condensation catalyst to form a primer film. Some condensation catalysts, including organotitanates, are part of the primer film and promote bonding between the primer and adhesive.
Theoretically, the best primer film is a monomolecular layer, with the compatible group facing the substrate and the organic groups facing the organic silicone adhesive surface. In reality, these monolayers don't exist, but compatible bi- or trilayers do. This illustrates the importance of thin primer films and the necessity of solvent carriers in the primer formulation. Thick, overly primed surfaces tend to build chalky primer films that can result in points of adhesive failure.
Application methods range from simply wiping the primer on a surface to spraying the primer through a paint-type sprayer. The primer is applied in a thin, uniform film, allowing the solvent to evaporate and the reactive groups to hydrolyze and condense into a film. The goal is to produce an even film, with no pooling or fisheyes.
After the solvent evaporates, there must be 30–60% humidity in the air. However, too much water slows condensation. The usual recommended minimum time for a primer to cure, from application to usage, is 30 minutes, although it is possible to accelerate the primer cure process with heat from 35° to 80°C. If a heat cure is used, careful experiments must be performed to ensure the primed adhesion doesn't suffer from this process. The primed surface should last a long time, provided it is protected from contamination or abrasion.
Primers are moisture-sensitive, and poor handling of the bottles can affect performance. If the bottles are opened repeatedly, efforts must be made to prevent the introduction of moisture into the bottle. Humid room air can be displaced with either dry air or inert gases such as nitrogen. Another method is to package the primer in the smallest size practical, thus minimizing the number of times any particular bottle is opened.
An additional application consideration is residue build-up on applicators or spray heads. Primers tend to form chalky residues that can be transferred to parts when using old applicators. Spray heads can be partially or completely obstructed by residue. Some production-floor controls, such as changing applicators periodically during the day or inspecting spray heads every day, are required to combat this issue.
To promote adhesion to novel substrates, a unique primer, SP-270, was developed. It contains a proprietary blend of silanes, catalysts, and solvents with a low surface energy to provide better wetting. This blend also increases the wet out between the silicone adhesive and primer layer.
|
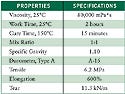
Table II. (click to enlarge) Table II. MEDI-4013 typical properties. |
Adhesives
MED1-4013, a fast-cure addition-cure adhesive, was used with the SP-207 primer (see Table II). The cure mechanism of this addition-cure system involves the direct addition of the hydride functional crosslinker to the vinyl functional polymer, forming an ethylene bridge crosslink (see Figure 8). Because this mechanism involves no leaving group, unlike the one-parts, these systems can cure in closed environments.
|
Figure 8. (click to enlarge) Figure 8. A platinum-cure mechanism. |
Some platinum systems can fully cure at room temperature in 24 hours or can be accelerated with heat. They can be partially cured, tack-free, with heat and then packaged. Curing continues in the sealed package with no adverse effects. Special care to eliminate the presence of contaminants that might have a negative effect on the catalyst may be necessary. Materials to avoid are typically sulfur compounds, nitrile, and unreacted vinyl groups.
Treatments
During the research, the difficult substrates, such as polycarbonate, polysulfone, polyetherimide, and polyimide, show little improvement in tensile strength, even when cleaned and primed. Abrasion or solvent etching techniques were evaluated, but modification of the bonding sites was more effective with flame treatment.
Flame treatment uses a propane torch to oxidize the substrate's surface, resulting in a high-energy surface that is conducive to bonding. The flame generates excited species (radical oxygen molecules), which attack the polymer surface. The flame may also burn off the absorbed water that occupied the reactive groups on the surface. Care must be taken not to overheat the surface and cause damage; a cooler flame is a better solution to prevent polymer damage. Analysis indicated the presence of alcohol, acidic, and carbonyl groups present on the surface of the polymers. Flame treatment may also oxidize any hydrocarbon-type contaminant.
Plasma treatment, or the deposition of specific reactive groups on the substrate's surface, can also improve bondability and adhesion. After treating and priming a substrate, the resulting bond is generally stronger.
Testing Parameters
|
Figure 9. (click to enlarge) Figure 9. A diagram of the lap joint. |
Each substrate was cut into a lap-shear configuration, 1 in. wide by 4 in. long. Six strips of each substrate were prepared to make three test panels. Panels were cleaned with isopropanol to remove dirt, grease, or particulates. SP-270 was added to a 1-sq-in. area on one end of each lap panel as described above and left to sit for at least 30 minutes. A bond thickness target of 5 mil (0.005 in.) was used for applying the MED1-4013 to the primed area of the panels. The two panels were pressed together, forming a sandwich (see Figure 9). Researchers made sure not to apply too much pressure over the bond surface.
Sandwiched panels were placed in an air-circulating oven set at 70°C for a one-hour cure. ASTM D-1002, “Apparent Shear Strength of Single-Lap-Joint Adhesively Bonded Metal Specimens by Tension Loading,” was used as a test method reference.
Difficult substrates were flame-treated, then primed with SP-270 silicone. Passing a propane torch over the surface of the substrate treated difficult substrates; but care was taken to not damage or degrade the substrate due to excess localized heat.
All plasma-treated substrates were sent to Plasma Etch Inc. to be treated. These panels were plasma treated at 38°C for 15 minutes at 350 W, using oxygen at 120 cm3/min as the plasma gas. These panels were then wrapped in plasma-treated aluminum foil, vacuum-sealed in plastic, and shipped back to the laboratory. Half of the surfaces of the plasma-treated substrates were primed with SP-270 silicone primer, and half were left unprimed.
The equipment used to test for lap shear value was an Istron Model 1011, with MTS data acquisition and a 454-kg (1000-lb) load cell installed.
Conclusion
|
Figure 10. (click to enlarge) Figure 10. Mean lap-shear results, compared with primed material. |
Across the board, breakdown mechanisms in adhesively bonded joints were caused by substrate, adhesive, or cohesive failure.
Substrate failure is the fracture failure within the substrate, indicating that the bond is stronger than the substrate. Adhesive failure is the interfacial failure between the adhesive and the substrate, indicating a weak boundary layer, often from improper surface preparation or adhesive choice. Cohesive failure is the internal failure of the adhesive itself. This indicates that the strength of the bonded materials is greater than the strength of the adhesive's own physical properties. Usually, joint failure is neither completely cohesive nor completely adhesive. Measurement of the success of a particular joint is based on the relative percentage of cohesive-to-adhesive failure.
|
Figure 11. (click to enlarge) Figure 11. Mean lap-shear results, compared with flame- and plasma-treated materials. |
For this silicone adhesive and primer system, cohesive failure with all the primed and treated substrates was most often observed (see Figure 10). This is ideal for applications that require a hermetic seal or a bond that can be reworked or repaired as necessary. Although the lap-shear strength is lower with some substrates, such as titanium and urethane, these adhesive systems work for applications requiring the most basic adhesion. The percentage of adhesive versus cohesive failure in the bond line is usually higher for these materials.
Untreated and unprimed materials tended to exhibit mostly adhesive failure. Although priming or treating a substrate adds an extra step to the manufacturing process, it may often be necessary when working with unusual or difficult substrates.
The plasma treatment of a surface, when combined with a chemical primer, may provide the optimum tensile lap shear results on certain substrates; but, as the data show (see Figure 11), there is no obvious way to develop a product without systematic bench testing to determine the best adhesive system and technology. Variables, such as the type of gas used, temperature, and dwell time, may affect the process and results.
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like