Originally Published MDDI July 2006 COATINGSThe next generation of drug-eluting stents may include improvements on drugs, new stent designs, new stent materials, and coating technology.
COATINGS
|
Figure 1. Colorized scanning electron microscope (SEM) image of a biodegradable drug-eluting coating on a coronary stent. |
Catheter-based interventions have revolutionized the practice of cardiovascular medicine worldwide. As a substitute for open-heart surgery, these techniques have greatly improved outcomes in the treatment of patients with cardiovascular disease and have reduced patient recovery times. Typical catheter-based treatments are often outpatient procedures or require only a single night's hospital stay.
Metallic stent technology has been at the forefront of this catheter-based treatment revolution. It began with bare-metal stents fabricated from stainless steel and has continued more recently with the advent of drug-eluting stents (DES). Now, as medical device developers move beyond durable polymers in the development of stent technology, they must address both the engineering and clinical needs of existing DES systems. These needs should guide the next generation of DES technology.
Bare-metal stent technology was successful because it immediately restored circulation in the affected vessels. However, the long-term success (longer than six months) of the treatment was not always optimal. Many bare-metal stents required reintervention for restenosis, a premature closing of the treated vessel. The results of peer-reviewed double-blind studies indicate that reintervention occurs in more than 15% of vessels treated with a bare-metal stent but in less than 5% of patients treated with DES systems.1
The irritation of the vessel caused by the stent placement triggers a proliferation of smooth muscle cells in the blood vessel. These proliferating muscle cells can cause the vessel to occlude or cause the stent to collapse. By either mechanism, the narrowed vessel lumen reduces flow and heightens the risk of a cardiac event in the patient. However, the sheer number of bare-metal stents placed each year, more than 1 million, demonstrated the scope of the problem. The only reasonable solution was an improvement in the technology itself. The restenosis issue had to be addressed to ensure the long-term success of the treatment modality. DES technology that utilized durable polymer systems to release drugs proved to be the winner.
Clinical Perspectives
At the core of DES technology is a conventional metal stent that props open the vessel while delivering a drug from a polymer coating. The eluted drug prevents the proliferation of smooth muscle cells. The bare-metal stent is coated with a polymer containing the drug to be delivered (see Figure 1). The biocompatible polymers currently available on stent systems are of a durable nature, meaning that they remain on the stent for the life of the implant.
To date, two types of drugs have been commercially available on durable polymer based DES systems: sirolimus and paclitaxel. In the United States, Cordis sells a sirolimus-eluting stent (Cypher) and Boston Scientific sells a paclitaxel-eluting stent (Taxus). The clinical success of these technologies has been dramatic: The restenosis rate in patients receiving DES is less than 10%, dramatically reducing the reintervention rate and cost of therapy while improving long-term outcomes. These stents have been implanted in more than 3 million patients. The many positive outcomes using this technology (lower restenosis rates and subsequent lower intervention rates) have made this technology highly successful in hospital cardiac catheterization labs.
Future DES Technology
Durable biocompatible polymers have enabled the key drug-delivery feature of this breakthrough technology. Given the success of the current generation of drug-eluting stents from both a clinical and a commercial perspective, the bar will be quite high for subsequent generations.
For engineers looking to develop future DES technology, many research and development activities are under way. In an attempt to offer differentiated products, researchers are exploring the possibility of incorporating drugs such as estradiol, dexamethasone, and pimecrolimus into future DES systems. Each compound has different mechanisms of action that are being studied in clinical trials to determine whether advantages exist over commercially available products. Other research activities under way in the field include the development of new coating technologies like biodegradable polymer systems. Although durable polymer-coated stents are suitable for the delivery of small hydrophobic drugs (such as those mentioned earlier), these polymers are unlikely to be amenable to next-generation therapeutics, such as peptides, proteins, or even cells. These agents may offer the benefit of enhanced endothelialization of stents and healing of the blood vessel. Because of their large size, proteins and peptides may be difficult to deliver by a diffusion-based process. With molecular weight being a rough proxy for physical dimension, sirolimus and paclitaxel are very small, with a molecular weight of roughly 1000 amu. However, most therapeutic proteins exceed a molecular weight of 50,000 amu, and cells are measured in microns. Therefore, one possible advance in DES technology may come in the form of new delivery vehicles.
From a clinical perspective, low restenosis and reintervention rates must be duplicated in any new coating technology. Additionally, any new drug-delivery coating must be compatible with the currently used pharmaceuticals as well as the next-generation compounds mentioned earlier. However, it is unlikely that the medical device industry will move away from the clinical experience of more than 3 million implants with the current drugs any time soon. These drugs have a known mechanism of action and a well-understood safety profile. Any new drug-delivery technology will likely continue to use these pharmaceutical agents into the next generation or perhaps even longer.
Future generations of commercial DES systems may include stents fabricated completely from biodegradable matrix-forming reagents. Issues faced in the development of such a stent design include the need for the polymer matrix to degrade in such a way that it will maintain its mechanical integrity until the vessel is completely endothelialized. The stent must also provide sufficient radial force to open stenosed vessels. It may be possible to design biodegradable devices that have shape memory such that, when formed into specific shapes and configurations, could be altered by a dehydration process to facilitate delivery into the body. The device would then return to its original shape when rehydrated in vivo. The formed device could have the therapeutic agent integrated into the matrix, which would then be released into the body through the biodegradation process.
Requirements for New Coating Technologies
|
Figure 2. (click to enlarge) Accelerated elution data comparing the sirolimus-delivery profiles of two biodegradable polymer matrix coating formulations with that of a durable Bravo drug-delivery polymer matrix coating. |
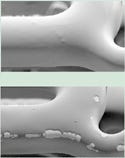
The performance expectations for new drug-delivery coatings will be quite high. Several requirements must be met. A new system should match the drug-elution profiles for the current sirolimus and paclitaxel systems (see Figure 2). Matching these profiles preserves the integrity of the clinical experience with these drugs and may reduce the regulatory burden for approval of a new delivery technology. If the new coating is biodegradable, it should degrade within six to 12 months. The degradation process must not affect the viability of the drugs being delivered. The chemistry of the degradation must not introduce any local acidity that would alter the chemistry of the therapeutic agents. In addition, the degradation process must not produce any physiologically significant emboli (particulate) (see Figure 3). Regarding this requirement, the width of a stent strut in current metal stents is 100–150 µm. If a piece of the coating the size of a strut were to detach, it could lead to thrombosis downstream.
|
Figure 3. Coronary stents coated with an enzymatically degrading coating in a mock physiologic fluid. Stent (a) at t = 0, stent (b) at t = 7 days. Note the erosion of the biodegradable polymer coating from the stent surface. |
Once a biodegradable drug-delivery coating has been conceived, it must also meet the many requirements of the medical device manufacturing environment. Manufacturing requirements for new coating technologies include the following:
• Ability to work with current deposition technologies.
• Compatibility with most of the current stent manufacturing processes, such as stent loading, crimping, and sterilization.
• Compatibility with existing manufacturing work flow.
The use of current durable polymer manufacturing technology would allow DES manufacturers to continue to reap financial benefits from their substantial investments in assets. Device companies will be looking to create the next-generation DES technology that meets end-user requirements to facilitate sales success, is compatible with current internal processes, and is flexible with an eye toward future development.
Next-Generation Technologies
The success of the current technology argues for an incremental approach rather than a revolution. Given the clinical and mechanical specifications that such technologies must meet, it is interesting to review the technologies that are under consideration. Many methods are being considered in the race to determine the next step in DES technology. Options include new polymer-coating technology, new stent designs and materials, and completely new approaches such as drug-coated angioplasty balloons. In fact, calcified plaque management may ultimately involve drug-delivering angioplasty balloons or fully biodegradable stents. In the next few years, metal stents featuring biodegradable drug-delivery polymer coatings may become available in the United States. But the requirements discussed above must be met.
From a polymer coating perspective, systems currently in development feature a variety of chemistries. Developments are under way at the major medical device companies as well as at specialized polymer-coating development companies. New systems have a high bar to clear to satisfy the clinical needs of physician end-users and their patients, and the possibility of new and challenging regulatory requirements exists. New technologies must prevent restenosis by delivering the pharmaceutical in a clinically relevant manner, accelerate the endothelialization process by degrading quickly after delivering the drug payload, and not introduce any new clinical problems that could potentially cause a cardiac event unrelated to the underlying disease. The next three to five years are expected to see active research and development in the DES field and the potential commercialization of exciting new technologies.
Brian L. Robey is vice president and general manager, drug delivery, at SurModics Inc. (Eden Prairie, MN). John Buan is marketing manager at SurModics and can be reached at [email protected].
References
1. JW Moses and MB Leon, “Clinical Results of the SIRIUS Study,” in Proceedings of the Transcatheter Cardiovascular Therapeutics Conference (Washington, DC: Cardiovascular Research Foundation, 2002).
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like