August 25, 2015
A researcher at Penn State has helped develop promising citrate-based biomaterials.
Kristopher Sturgis
|
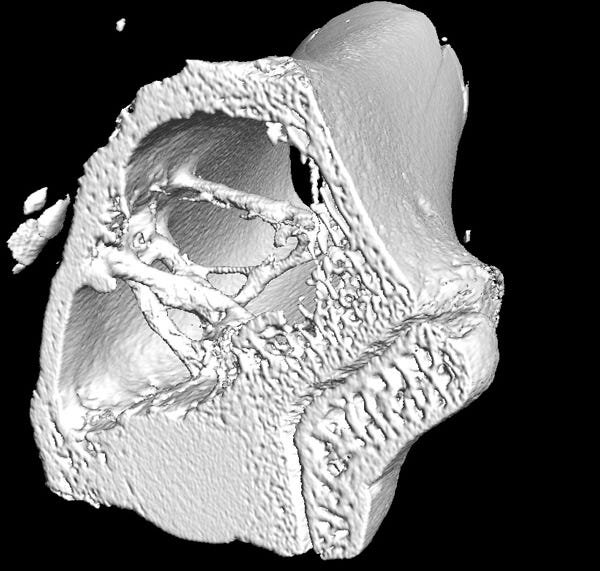
Citrate materials can promote osteo-integration and new bone formation, according to the Penn State researchers. This photo demonstrates that a citrate bone implant prompting bone regeneration and fully integrating with the surrounding bone. (Image courtesy of Jian Yang, Penn State) |
Biomaterials are the foundation for medical technology innovation, says Jian Yang, PhD, biomedical engineering professor at Pennsylvania State University (State College, PA). He should know: his lab is working on unique citrate-based biomaterials and molecules that can be used for an array of medical applications. "The most promising materials so far in our lab are citrate bone biomaterials and fluorescent polymers," Yang says, who is scheduled to speak on the topic at MD&M Philadelphia on October 8.
The citrate materials are multifunctional. They exhibit antimicrobial properties to combat infection problems that are common in the use of implantable medical devices. They boast tunable mechanical properties and can be as strong as human cortical bone for load-bearing applications. In addition, they promote osteo-integration and new bone formation and possess intrinsichemocompatibility to presentblood clotting in blood-contacting applications such as blood vessel replacement and vascular stent coating.
|
Jian Yang |
"The citrate chemistry is robust and extremely convenient for the design and synthesis of materials with different functionalities such as wet-tissue adhesive properties and photo-luminescent properties," Yang says. "I would expect we will see citrate orthopedic products in about 2-3 years."
Yang's lab has also created a new family of citrate-based fluorescent polymers and small molecules that can potentially be used in medical imaging applications. "We have fabricated fluorescent nanoparticles that can specifically target and deliver therapeutics to tumor cells for prostate cancer imaging, diagnosis, and treatment," he says.
In the following Q&A, Yang sheds more light on the novel citrate-based materials, which have been licensed to a startup known as Acutiive Technologies Inc. (Allendale, NJ) for orthopedic applications.
MPMN: What sort of medical devices do you see most benefiting from your citrate-based materials?
Yang: Based on newly developed cross-linked urethane-doped polyester elastomers (CUPEs), we are fabricating biodegradable nerve conduits for peripheral nerve regeneration and blood vessel scaffold for small-diameter blood vessel regeneration in additional to the above mentioned orthopedic devices such as biodegradable bone screws, plates, and spinal fusion devices. We are also developing bio-glue products that can be used for wound management in surgery.
MPMN. What do you see as the biggest materials-related challenge that medical device engineers are facing these days?
Yang: The challenge is to develop dynamic biomaterials that can respond to the biological needs to fulfill the necessary biochemical, biological, and biomechanical functions at different timing and stages of the lifespan of medical devices. For example, for load-bearing orthopedic devices such as a spinal fusion disc, it would be optimal that the spinal fusion disc can maintain significant strengths before significant bone tissue formed for spinal fusion. This requires the designed materials for spinal fusion disc fabrication should degrade slowly in the beginning to maintain significant strength but speed up at later stages to leave rooms for tissue replacement.
Controlling degradation to match tissue formation is not trivial in tissue engineering.
We have recently developed citrate-based biodegradable elastomers via click chemistry that can show an interesting first-slow-then-fast two-stage degradation behavior.
MPMN: Do you foresee any challenges in the large-scale production or manufacturing processes for these biomaterials?
Yang: One of the huge advantages of our citrate biomaterials are that they are fabricated via a cost-effective and convenient manufacturing one-step condensation process that allows us easily scale up the production of our biomaterials.
MPMN: What do you see as the main drawbacks to conventional medical device materials?
Yang: Current biomaterials do not solve all the existing problems in medicine. Yet biomaterials are a huge field. There are so many new materials that have been developed, or are being developed, but very few have reached the market.
In the field of biodegradable polymers, there are only a handful polymers used in FDA-approved devices. Those polymers certainly cannot meet the ever-growing need in diversified medical conditions.We should be open to seeking new materials to solve unmet clinical problems. But the new materials should be scrutinized for their safety and functions before they can be used in medical devices for humans.
Acuitive Technologies are working to commercialize our citrate bone biomaterials in orthopedic applications. Significant preclinical studies are needed that requires significant funding to support. It is expected that the first citrate orthopedic device product will be available in two to three years.
MPMN: Many of the polymers used in the medical device sector were originally developed for other non-medical applications. How much is this changing?
Yang: We are in a designed material age. Existing materials may not meet the requirements to solve many global medical challenges. We will have to rationally design new materials that are not previously existed to solve the medical problems. This requires coordination and collaborations from a team of scientists, engineers, and clinical practitioners with complementary expertise. Tissue engineering is a perfect example or field that requires close collaborations from material scientists, chemists, biologists, engineers, and clinicians for clinical translation.
Hear Dr. Yang speak and learn more about cutting-edge medical devices at MD&M Philadelphia, October 7-8. |
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like