MATERIALS
January 1, 2007
|
Figure 1. (click to enlarge) Available in tape or rod form, carbon-fiber composites are increasingly being used in place of metals for implants. |
The basic mechanical principles behind implants used for total joint arthroplasty are substantially unchanged since the development of low-friction hip implants pioneered by Sir John Charnley and his team at Wrightington Hospital (Lancashire, UK) in the early 1960s. Primarily, implants developed at that time involved the use of a strong and stiff metal element to carry the load, combined with a polyethylene-and-metal wear-couple bearing. This combination is still the basis for most current designs, although improvements in design, fixation, and materials have led to increased implant life span and improved performance.
Initial improvements included new grades of ultra-high-molecular-weight polyethylene (UHMWPE) and the application of irradiation treatments to induce cross-linking with consequent increased wear resistance. New metal alloys helped reduce implant stiffness and made implants closer to the properties of real bone. Implant fixation techniques were improved to eliminate the need for polymethyl methacrylate cement. Modern designs feature some or all of these advances. Further advances have been made with respect to the bearing elements, such as the development of ceramic-on-ceramic and metal-on-metal designs that improve wear performance and increase service life. The use of these alternatives, however, is less common than UHMWPE.
Although metals remain a key material in the development of implants, their use can present significant challenges. For example, stiff metallic implants can assume the applied load that is normally placed on the adjoining bone. Shifting of the load to the implant can result in stress shielding, a phenomenon that can lead to bone loss and a weakening of the implant site. A less stiff implant may allow better load sharing, reducing the effect of stress shielding. New developments in carbon-fiber-reinforced (CFR) polymer composite materials have made the material an attractive alternative to the metallic materials traditionally used in implant development.
Carbon-Fiber-Reinforced PEEK Composite Materials
CFR polyetheretherketone (PEEK) composites offer design freedom not readily achievable with other biomaterials. Recent increases in material availability to the implant market make CFR composite more accessible. The use of these materials for new device development is increasingly popular, possibly because of the increase in polymer use in the spine, where CE-marked and FDA-cleared implantable devices made from PEEK are used regularly.
PEEK has good mechanical and biological durability. It is compatible with imaging devices, especially magnetic resonance imaging, and it is easily fabricated using molding or machining technology. Manufacturers can increase the material's strength, stiffness, and impact properties by adding chopped carbon fibers or, more substantially, by introducing continuous fibers at high concentrations.
Using PEEK as a base polymer matrix in combination with carbon fibers for medical implants is not a new concept.1 Laboratories began studying a range of carbon fiber and polymer matrix composite combinations for implant applications in the late 1980s. These included polysulphone, polymethylmethacrylate, and epoxy polymer matrices. The composites were considered viable alternatives to commonly used cobalt-chrome and titanium alloys because of their closer modulus matching with bone, giving rise to more efficient stress transfer.
However, these materials were not entirely successful. In vivo environments were hard on the composites and they required repeated sterilization. Considerations of durability, toughness, and biocompatibility, particularly following sterilization and aging, led to the proposal in 1990 that PEEK would be a better choice of base polymer.
It has only been within the last 15 years that the medical industry has had access to medical-grade PEEK for human-implantable devices. Carbon-fiber grades based on 30%-by-weight short fibers have been evaluated and approved for use in spinal applications.2 Compared with metals, the short-fiber approach has mechanical strength limitations that make it unsuitable for high-load-bearing applications such as hip implants. In at least one hip design, however, these limitations have been overcome using a hybrid approach in which CFR PEEK composite works in combination with a metallic component.3 Such a formula provides an elastically tailored implant that can reduce stress concentrations in the medulla and reduce stress fractures in the hip prosthesis.
A PEEK-based preimpregnated carbon-fiber-composite tape material has been developed with a fiber content of approximately 62% by volume, providing high levels of mechanical performance and substantially bridging the gap between polymers and metals. The material offers strength and stiffness performance similar to those of commonly used metallic implant materials. The actual properties of this composite material are controlled by the orientation of the constituent fibers, which is dictated at the design stage and set during manufacture. This material is available as tape or in rod form(see Figure 1).
|
Figure 2. (click to enlarge) Relative elastic modulus of a particular blend of CFR PEEK polymer and implantable metals. Cortical bone is approximately 15–20 GPa. |
Mechanical Properties. Because of its tensile strength, stiffness, and fatigue behavior, CFR PEEK composite can be used as a metal replacement in structural implants. Moreover, strength and stiffness can be tailored to meet or exceed those of metals. Figure 2 illustrates the relative stiffness properties of CFR PEEK compared with common metallic implant materials.4
CFR PEEK is available in several forms, including a unidirectional form in which the fibers are aligned along one axis (the principal stress direction) and a multidirectional form, meaning that the fibers are arranged in a multiangular, planar configuration. In this configuration, subsequent layers are offset, for example at 45º or 90º, to the reference axis, giving more-balanced properties in different in-plane directions.
Given that the stiffness of cortical bone is approximately 15–20 GPa, it is apparent that multidirectional CFR PEEK composite is similar to the stiffness of bone, more so than, say, titanium alloys.
|
Figure 3. (click to enlarge) Relative tensile strength of a particular blend of CFR PEEK polymer and implantable metals. |
In terms of strength, CFR PEEK composite again shows comparable performance to metallic materials, as illustrated in Figure 3.4 A tensile strength in excess of 900 MPa for multidirectional material compares favorably with commonly used implant metals such as titanium 6Al-4V, cobalt chrome alloy, and 316 stainless steel produced using a range of methods.
Design Flexibility. One of the chief advantages of CFR PEEK composite is the flexibility of design it offers medical device manufacturers. Fiber orientation, concentration, and composition, and the use of x-ray contrast media, can all be adjusted to accommodate the needs of the specific medical device application. For example, where maximum strength or stiffness is desired, fibers may be aligned in the directions parallel to the principal stresses, and the fiber composition and concentration can be optimized to achieve the desired performance. Alternatively, fiber composition, concentration, and orientation can be chosen to provide strength or stiffness closer to human bone and isotropic mechanical properties.
Part Fabrication. CFR PEEK composite preimpregnated tape can be processed using conventional high-temperature polymer composite processing techniques largely developed for aerospace structures and advanced automotive components. Processes can include autoclave molding, compression molding, filament winding, and pultrusion to create simple or complex part geometries with specific fiber orientations for the desired design. The prerequisite is a high-temperature processing capability above the melting point of the base polymer (typically 340°–400°C). Finishing operations allow the parts to be trimmed to meet final engineering tolerances.
Alternative special injection molding technologies and a process known as composite flow molding have been developed.5,6 These processes can accommodate automated production systems and parts, such as translaminar pins and screws, that are difficult to create with other production techniques.
Composite flow molding involves using a highly anisotropic (having the property of being directionally dependent, or showing different characteristics in different directions) starting material, such as an aligned fiber-reinforced rod. The material must be pushed into a mold tool at high pressure and temperature, forcing the fibers to take new orientations for the desired mechanical performance. Load-bearing medical implants developed using CFR PEEK composite include spinal cages, translaminar fixation pins, bone fracture plates, screws, and intermedullary nails.
Imaging Compatibility. One of the most significant benefits associated with CFR PEEK composite is imaging compatibility. Because it is radiolucent, CFR PEEK enables artifact-free postoperative imaging and the ability to view tissue and bone growth and repair through computed tomography and x-ray imaging techniques. Because it is nonmetallic, CFR PEEK composite also offers MRI compatibility with almost no artifacts and a clear image for clinical investigation.
|
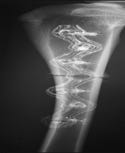
Figure 4. An osteosynthesis plate includes x-ray scattering materials that provide visibility. |
Parts made from CFR PEEK composite are normally radiolucent and virtually invisible to x-ray inspection. However, implants can be made visible by adding x-ray scattering materials, such as tantalum wire. Figure 4 shows an osteosynthesis plate bracing a broken bone. It is evident that the radiopacity has been tailored by design to allow visualization of the component with minimal screening of the fracture site. Importantly, x-ray opacity can be achieved in a way that also maintains clear MRI images.
Biocompatibility, Toxicology, and Sterilization
For any potential implant material, it is important to consider biocompatibility. Various composites of CFR PEEK are available; however, this section concentrates only on PEEK-Optima, because its testing history is known. Implantable-grade CFR PEEK composite material with continuous fibers is based on PEEK-Optima polymer. The material has been widely used and tested to meet the requirements of ISO 10993-6:1994, “Biological Evaluation of Medical Devices: Tests for Local Effects after Implantation.” It is critical to note that CFR PEEK can be formulated in a variety of ways and therefore it is possible that not all formulations of the material will meet the same standards of biocompatibility and toxicology.
CFR PEEK composite does not release leachables and thus does not affect the biological safety of the patient. The material has the following history:
PEEK-Optima polymer has already been comprehensively qualified and approved as a long-term implant material.
Carbon fibers have been used clinically for more than 20 years as a reinforcement component for implant materials without obvious leachable-related bioincompatibility reactions.7–9 The toxicological profile of carbon fibers is adequately characterized.
CFR PEEK-Optima has been tested in a cell-culture test system that allows a sensitive characterization of the solubility of materials. No cytotoxic effects were observed in the presence of extracts obtained under prolonged conditions for maximum sensitivity. These results indicate that organic and inorganic leachables are not released in cytotoxic concentrations.
The results of chemical analyses (gas chromatography) performed with the reinforced materials substantiate the unchanged inert properties of PEEK-Optima.
Implantable materials must also be compatible with modern sterilization methods. CFR PEEK composite has been tested in terms of mechanical properties and biocompatibility following high doses of gamma radiation (up to 73 kGy) and repeated steam cycles. The results of these tests show that the material is resilient to sterilization with almost no change in mechanical properties. Cytotoxicity studies and chemical analysis following solvent extraction show that the material does not release substances in cytotoxic concentrations and is considered safe following sterilization. The material is also listed with FDA.
Conclusion
CFR PEEK polymer is a high-quality, biocompatible material with a high fiber content and tailored fiber orientation distribution. It combines the high strength of metals with the extensive biocompatibility and imaging compatibility of polymers. The material has approved applications in the United States and Europe. The advantages of this material are its ability to closely match the modulus of natural bone while retaining high strength, good fatigue resistance, and compatibility with MRI, CT, and x-ray technologies.
Stuart Green, PhD, is the technical manager for Invibio Ltd. (Thronton Cleveleys, Lancashire, UK). Contact him at [email protected].
References
1. Kofi Boamah Kwarteng and Cas Stark, “Carbon Fiber Reinforced PEEK (APC-2/AS-4) Composites for Orthopaedic Implants,” SAMPE Quarterly 22 (Oct. 1990): 10–14.
2. M Moumene et al., “Carbon Fiber/PEEK Composite Stackable Cage Implants: A Biomechanical Evaluation,” in 27th Annual Meeting Transactions Society for Biomaterials (Mt. Laurel, NJ: Society for Biomaterials, 2001).
3. CA Scotchford et al., “Use of a Novel Carbon Fibre Composite Material for the Femoral Stem Component of a THR System. In Vitro Biological Assessment,” Biomaterials 26 (2003): 4871–4879.
4. John Brunski, “Metals,” in Biomaterials Science, An Introduction to Materials in Medicine, ed. Ratner et al. (San Diego: Elsevier Academic Press, 1996): 37.
5. J Mayer et al., “New Injection Molding Technologies for Carbon Fiber Reinforced Polyetheretherketone (PEEK) Applied to Highly Loaded, Anisotropic Implants,” Sixth World Biomaterials Congress Transactions 3 (May 2000).
6. R Leaversuch, “New Twist in Thermoplastic Composites Makes High-Strength, Net-Shape Fasteners,” Plastics Technology (August 2003).
7. R Bader et al., “Carbon Fibre-Reinforced Plastics as Implant Materials,” Orthopäde 32, no. 1 (2003): 32–40.
8. M Blazewicz, “Carbon Materials in the Treatment of Soft and Hard Tissue Injuries,” European Cells and Materials 2 (2001): 21–29.
9. JF Mano et al., “Bioinert, Biodegradable and Injectable Polymeric Matrix Composites for Hard Tissue Replacement: State of the Art and Recent Developments,” Composites Science and Technology 64 (2004): 789–817.
Copyright ©2007 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like