August 24, 2015
|
Demand in medtech is increasing for tiny tubing as well as for relatively large tubing. |
Medical extrusion is proving to be a dynamic field, says Raumedic's Steve Maxson. It's time to pay attention to this quickly evolving technological niche, which now offers medtech manufacturers unprecedented flexibility and control.
Brian Buntz
One might think that the production of medical tubing is fairly straightforward. But medical extrusion, now increasingly automated, requires sophisticated technology.
On top of that, the demands of medtech companies can vary greatly depending on the application. While many medtech OEMs are still requesting ever-smaller tubing, others are asking for tubing with relatively large diameters. Producing both types requires substantially different equipment and processes.
In addition, a growing number of companies are investigating the use of new medical tubing materials, including incorporating nano-sized taggant particles for security and anti-counterfeiting purposes, says Steve Maxson, a U.S.-based technical sales manager at Raumedic Inc. (Helmbrechts, Germany).
In the following Q&A, Maxson discusses a range of topics, including how in-line measurement systems offer closed-loop control of tubing diameter, discusses how structural heart sector is increasing demand for tubing as large as 24 French (Fr),and touches on Raumedic's U.S. expansion plans.
MPMN: How is automation technology redefining medical extrusion?
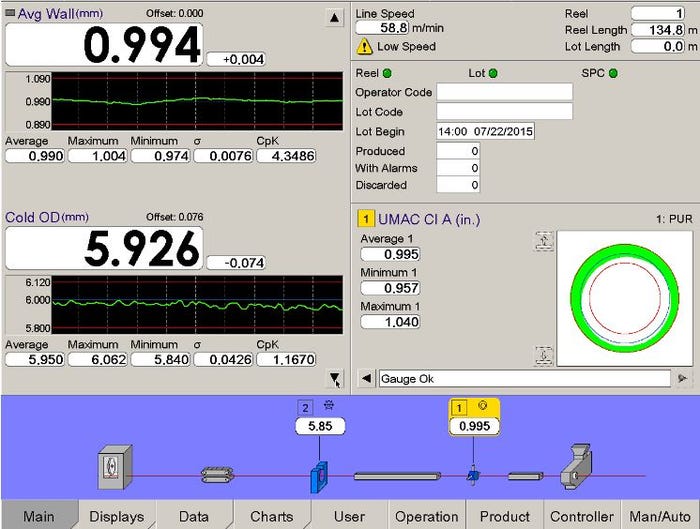
Maxson: A perfect example of how automation is used to refine medical extrusion is the use of in-line measurement systems for automatic closed-loop control of tubing OD and wall thickness, and for automated good/bad quality sorting. In a typical closed-loop controlled extrusion process, an OD gauge is used to measure and control the OD and an ultrasonic wall gauge is used to measure and control the wall thickness. If the OD readings are moving toward the lower or upper specification limits, the system automatically adjusts an extrusion control parameter to maintain the OD within tolerance. Likewise, if the wall thickness readings are moving off specification a different control parameter can be automatically adjusted to maintain the wall thickness. During the course of the extrusion run, if the tubing OD or wall thickness is out of specification, the measurement system controller can be integrated with the downstream cutting and conveying equipment so that "bad" tubes are discarded at the end of the extrusion line and "good" parts are blown off into a stainless steel tray.
|
Steve Maxson is a technical sales manager at Raumedic Inc. |
MPMN: Which kinds of medical device applications continue to drive the microextrusion trend?
Maxson: Let's use cochlear implants as an example where very fine wires or electrodes are used to electrically stimulate the cochlea. In this example, an electrode may include a 25 µm (0.001 in.) miniature wire that is composed of 80% platinum and 20% iridium (Pt-Lt). Platinum is used because of its resistance to corrosion and iridium is added because of its high tensile strength properties. These physical properties are very important for device functionality.
The fine wire is typically "dip coated" with an ultra-fine layer of PTFE, which is bio-inert and has excellent coefficient of friction and dielectric properties. For the dipping coating process the fine wire is submerged in a solvent based PTFE solution in a multiple-pass process until the desired wall thickness is achieved. Each pass requires a new set-up that can potentially damage the wire. PTFE coatings are cured at the relatively high temperatures (greater than 660°F or 350°C) over time, which can have an adverse effect on the mechanical properties of the underlying specialty miniature wires. This is especially true for Nitinol (nickel-titanium) fine wires. A continuous micro extrusion process has the potential to protect the physical properties of the miniature wires because extrusion is a single-pass process with a shorter residence time required to achieve the desired wall thickness. Also, different draw down ratios in the extrusion die tooling can be used to orient the polymer chains and improve the physical properties of the polymer jacket.
MPMN: As tubing diameters continue to shrink, are you seeing more medical device companies approach you for pediatric and neonatal applications?
Maxson: Yes, this is seen in intravenous (IV) catheters that require micro-tubing with three to six co-extruded encapsulated radiopaque stripes. IV catheter tubing is made from FEP or polyurethane for in-dwelling purposes. Neonatal IV catheters can be miniature in size down to 26 GA or 0.64 mm OD ×0.45 mm ID (0.025 in. OD ×0.018 in. ID). IV catheter tubing for neonates is a growing micro extrusion application, especially in Asia.
|
This image shows wall thickness and OD trend lines. Image courtesy of Zumbach. |
MPMN: Changing gears from the miniaturization theme, what can you tell me about demand for tubing on the other end of the Fr scale?
|
Large-diameter tubing used for structural heart applications. |
Maxson: I am glad that you asked because there has been a lot written over the years about the miniaturization of medical devices such as those used for neuro applications. While tubing and tolerances have certainly become smaller and tighter, we should also recognize the growth of large bore tubing that is used in the growing structural heart segment. Heart valves are fairly large in size compared to other minimally invasive devices. For instance, a delivery system for a transcatheter heart valve entering through the femoral artery may require tubing with an internal diameter up to 24 Fr (0.315 in. or 8mm) or more. This requires vastly different extrusion equipment and process expertise compared to that of 1 Fr (0.013 in. or 0.33 mm) micro-tubing used in a delivery system for a neurovascular application.
MPMN: How is medical device OEMs' demand for materials evolving for tubing applications?
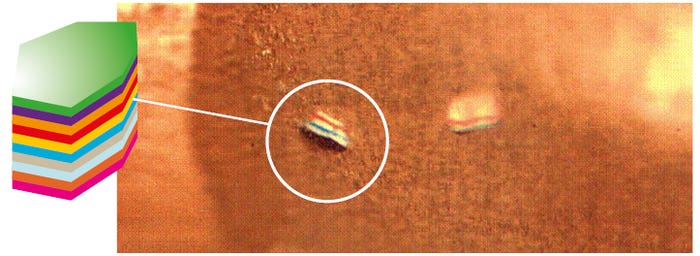
Maxson: Going beyond some of the common additives for medical compounds such as colorants, lubricants, antimicrobials, and radiopacifiers, what I find interesting is the addition of nano-sized taggant particles to medical-grade compounds for security and anti-counterfeiting purposes. Taggants are composed of multiple layers of a color to form a security code that is unique to the customer or application. The micro taggants can be added into materials at very low concentrations and they can be detected with a microscope or hand held device. Over the next several years, the FDA will be establishing a Unique Device Identification (UDI) system to identify devices with a specific code. Taggants can potentially also be used to authenticate the compounds that are used in tubing for medical devices.
MPMN: I understand that your firm is building a new plant in the United States. What level of growth do you see from your clients in the life science industry in the United States as compared with Europe and Asia?
Maxson: We see growth across the board in all geographic areas. To support the growth in Europe, we just completed construction of a new facility that is located right next to our head office in Bavaria. The new building includes cleanroom production and manufacturing facilities as well as laboratory and office space. The new U.S. headquarters will include a state-of-the-art clean room for the production of medical device components. Stay tuned for our plans in Asia.
|
Tiny micro tags are being used in tubing for security and anti-counterfeiting applications. |
MPMN: Your website says your new facility will combine the strengths of United States and German engineering. What do you think are the biggest strengths and of U.S. and German engineering?
Maxson: Germany is Europe's largest market for medical device manufacturing and German medical technology is the third largest in the world after the United States and Japan. In my opinion, German engineering takes a long-term, high-quality approach to device component design and manufacturing, which is highly beneficial for the stringent requirements within worldwide medical device market. The biggest strengths of German engineering is a highly skilled and well-trained workforce that has strong ties to universities and research institutes to support medical device developments.
The United States is the world leader in medical technology and a high percentage of medical device company revenues are devoted to research and development. There is also an active venture capital structure that invests in early stage medical device technology companies. This is in spite of a highly regulated and bureaucratic FDA approval process that results in significantly longer time to market compared to Germany.
Learn more about cutting-edge medical devices at MEDevice San Diego, September 1-2 and MD&M Philadelphia, October 7-8. |
Brian Buntz is the editor-in-chief of MPMN and Qmed. Follow him on Twitter at @brian_buntz.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like