Medical device companies willing to align their research activities with government objectives can enjoy increased product development opportunities and financial returns.
October 26, 2017
The U.S. government has a rich history of sponsoring innovative programs that bring about technological advances that benefit people everywhere. Prime examples include NASA’s space program, which led to products such as memory foam, advances in solar panels, and personal heart rate monitors, and DARPA advances that enabled internet communications and GPS devices. Although the complexity of intellectual property rights and government contracting can be intimidating, there are substantial potential benefits for companies who partner with the government in research and development.
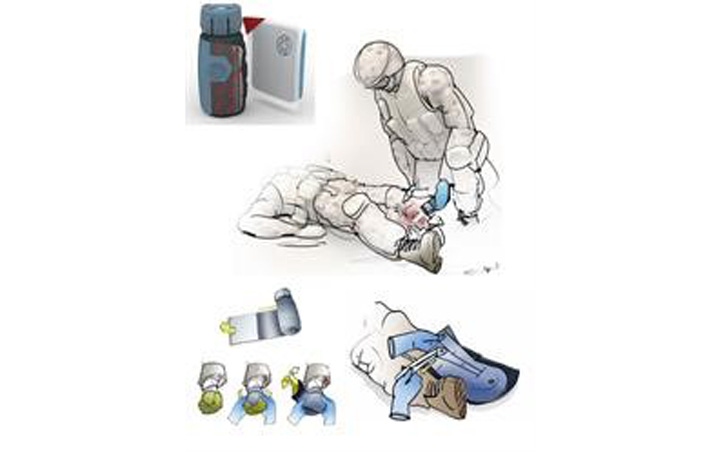
In the field of wound care, a new product to treat severely injured limbs on the battlefield has potential to assist diabetics suffering from foot ulcers. The Office of Naval Research sponsored a program to develop a medical device called the Acute Care Cover for Severely Injured Limbs, or ACCSIL, to preserve severely injured limb tissue. It is intended for use by corpsmen/medics/first responders at the point of injury with removal performed later by doctors and surgeons at definitive Medical Care Facilities.
ACCSIL is designed to preserve injured tissues in the best possible state for up to 72 hours after the time of injury. During ACCSIL development, there was a need to develop a way to deliver oxygen to tissue in a compact, lightweight form because when a tourniquet is applied to an injured limb, the tissue can become ischemic and further damaged. To address this need, an oxygen generating "pump" that does not require power or batteries and steadily provides three liters of oxygen to the wound site over three days was developed.
Although this pump can change the methods used to treat traumatic battlefield injuries and improve the overall quality of life for service men and women, it also has potential to aid in the treatment of other mainstream medical conditions, such as the ability to treat chronic pervasive wounds originating from diabetic ulcers. Over 30 million people in the United States have diabetes and between 10–15 percent will develop diabetic foot ulcers. The ability to provide steady, low levels of oxygen to these wounds without the need for electrical power or batteries could have long-term benefits for these patients.
The ACCSIL oxygen pump provides a good example of how technologies developed for the government can be leveraged for civilian and commercial markets, resulting in a positive impact to society. Working with the government to meet their pressing needs and unique applications can result in increased product development opportunities and financial returns for those companies willing to align their research activities with government objectives.
Hear from Battelle experts at upcoming UBM events. Stephanie Domas, lead medical product security engineer at Battelle, will be discussing FDA 510(k) cybersecurity requirements at MD&M Minneapolis on November 9. Megan Moore, program director at Battelle, will discuss how to evaluate new technology with a balanced score-card approach at BIOMEDevice San Jose on December 6. Use code "MDDI" to get 20% off conference registration.
About the Author(s)
You May Also Like


