March 2, 2015
Making its debut at MD&M West, EG GILERO's medtech roots stretch back decades.
Brian Buntz
|
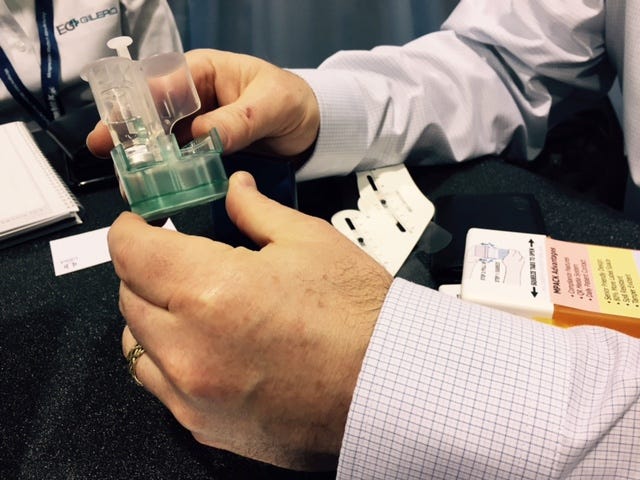
EG GILERO assisted in the development of this drug-delivery device, which cut the steps needed to deploy a lipolyzed drug from five to three. |
Newcomer EG GILERO is no stranger to the medical business. The company was blended from the merging of GILERO Biomedical, Medacys, InField Medical, as well as the medical businesses of eNNOVEA and ValTech Holdings.
Headquartered in Durham, NC, the company has U.S. facilities stretching from Florida to New York, EG GILERO offers a suite of contract manufacturing offerings and related services. "But the thing that makes us different from being a straight contract manufacturer is that we provide regulatory support," says Tim Hopper, the company's chief marketing officer. "We submit and write 510(k)s and are used to going to the FDA and getting quick turn around. Our average time for a Class II predicated 510(k) is about 45 days, which is about half the time it normally takes."
Solely focused on the medical market, the company offers assistance with the entire product development cycle, from design through to commercialization. It also helps pharmaceutical clients develop packaging and refine their drug-delivery technology.
Also unique is the fact that the company has first-hand experience with commercializtion. "We don't want to necessarily compete with any of our customers, but I think it shows that we know what it is like to get a product to market." So the company's logic is: if we can commercialize medical products ourselves, we can help other medical technology companies do it as well.
At MD&M West, one of the examples of the technologies the company has helped develop was a drug reconstitution device from Yukon Medical (Durham, NC). Drugs are getting more exotic and they are getting lipolyzed. "You take out all of the fluid of the drug and it becomes a powder. The benefit of having a drug is that it has a limitless storage capability," Hopper says. "As soon as you have fluid in it, that is where you have expiration dates on drugs."
The device only requires three steps to deliver the drug. The previously developed technology required the five steps from the user.
Refresh your medical device industry knowledge at BIOMEDevice Boston, May 6-7, 2015. |
As MD&M West draws to a close on February 12, check back on our blog for more show-related coverage.
Brian Buntz is the editor-in-chief of MPMN and Qmed. Follow him on Twitter at @brian_buntz.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like