Consider these manufacturing challenges faced by original equipment manufacturers working to commercialize innovative technologies in the IVD sector.

Modern medical practice and healthcare management are impossible without the vital role played by in vitro diagnostics (IVDs). They simplify the process of capturing, analyzing, and ultimately managing the biological complexity that underlies human disease. It’s estimated that IVDs influence roughly two-thirds of clinical decision making, yet account for only 2% of healthcare spending. 1
A range of innovative technologies and capabilities are transforming how we work with IVDs, including developments in molecular biology, miniaturization, and automation and in combination with digital healthcare platforms. As value-based care (VBC) becomes the new treatment paradigm, these medical devices are providing pathways to greater insight and helping to usher in the promise of more preventative, precise, and personalized medicine.
Diagnostic Insight—Why, Where, and When
At its most basic premise, precision medicine leverages genetic and genomic data to improve health outcomes with specific, personally tailored treatment plans and therapies. People are eager to take health and wellness into their own hands; they have questions: Am I sick? Are you sick? Is this treatment working? What treatment might work best…for me, or for a loved one?
Novel IVD technologies—many of which have been in the spotlight during the COVID-19 crisis—include polymerase chain reaction (PCR) testing, isothermal amplification testing, next generation sequencing (NGS), and immunoassays, like lateral flow testing. They all support this central impetus at the heart of diagnostics: the desire for insight.
Knowledge is power, and once we have a little, we tend to want more. The same goes for convenience and ease of use. This has led to accelerating the point-of-care and point-of-need trends within healthcare toward simpler, convenient, patient-focused solutions that help to better support personal health management.
In other words, we want diagnostics testing systems that are less dependent on a hospital, clinic, or central lab, but closer to the patient—safe, convenient, and ready to do their job where and when they are needed.
Challenges and Opportunities for IVD Manufacturers
Here are some of the key technology advances, manufacturing challenges, and opportunities impacting OEMs seeking to bring novel IVD technologies to market:
Sequencing is getting faster and less expensive; labs can now sequence a human genome in a single day.
Genomics-based workflows require the highest levels of reliability and precision—to get a molecular level of specificity, rapid molecular tests incorporate complex circuit boards, liquid reagents, lyophilized beads, microfluidic channels, and other advanced processes.
Driven, in part, by broader public familiarity, demand is increasing for robust and accurate biochemical test results available at the point of care, facilitating convenient and personal health management.
Balancing rapid adoption of novel technologies and time to market pressures must be accommodated within an evolving regulatory landscape, all while maintaining the highest quality standards.
In research provided by Grand View Research, the global market size of the in vitro diagnostics market in 2020 was estimated at $83.4 billion. For 2021, it’s estimated at just over $87 billion, with a compound annual growth (CAGR) of 4.5% between 2021 and 2027. Within this market, the largest segment (both worldwide and in the United States) is global reagents, with a market estimated at $55.3 billion in 2021.
To meet the opportunities in these and related markets, OEMs are increasingly outsourcing not just production but technical services for strategic cost containment.
Molecular Point-of-Care Testing (POCT)—Life After COVID
Certainly, the COVID-19 pandemic continues to drive urgency for robust tools that will aid in monitoring and managing containment efforts for infectious diseases beyond the current pandemic. IVDs’ essential role within public health has never been more visible than in today’s crisis. Molecular diagnostics testing has proven capable of being deployed at massive scale in the delivery of reliable and trusted results with short turnaround times at low cost.
It’s important to note, however, that enthusiasm in molecular diagnostics’ broad spectrum of technologies pre-dates COVID-19 by many years. At present, OEMs are expanding their testing portfolios and accelerating investment toward additional promising applications, such as liquid biopsy, markers for cardiac disease, fertility treatment program improvements, and other viruses, like sexually transmitted infections.
The underlying technologies have been in development for many years and are hitting their stride, as production processes continue to optimize delivery of these technologies at increasingly lower costs.

Companion Diagnostics—Improving Insight and Precision
Advances in molecular technology have also driven exceptional growth in companion diagnostics (CDx), which are in vitro devices that provide information essential for the safe and effective use of a corresponding therapeutic product.
The companion diagnostic helps unlock insights utilizing techniques such as real time PCR and NGS (via amplification and decoding of genetic and epigenetic information) for better understanding of the specific genes, biomarkers, and other biomedical factors impacting an individual's potential to acquire disease or be effectively treated for it.
The ability to determine whether a patient may or may not respond to a given treatment helps to better ensure the best treatment and medicine for that patient without wasting precious time with trial and error. In this way, CDx facilitates precision medicine and are invaluable for aligning healthcare outcomes more effectively with value-based care (VBC) efficacy models.
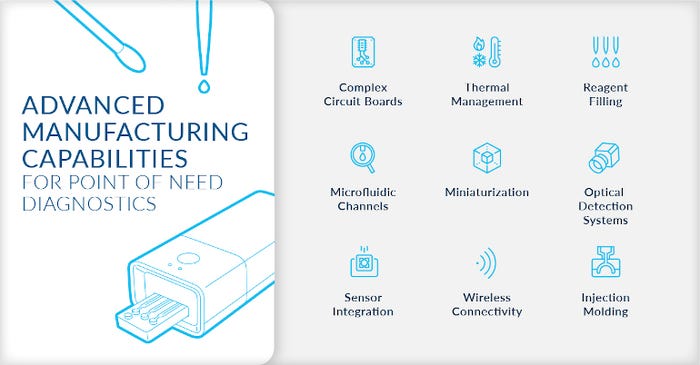
What Does It Take to Make a Molecular Test?
Across all medical device sectors, technical requirements tend to align along two streams: device-enabling technologies that generate device functionality, such as indicating disease presence, and commercial technologies, including what it takes to manufacture and successfully commercialize the device.
Today’s molecular point-of-care tests incorporate cartridges that interface embedded optical systems for indicating the presence of disease by detecting target genetic material (for example, the N-gene of SARS-CoV-2) through changes within an optical assay. Specialized technologies like surface modification, (which includes spotting and electrowetting), lyophilization, pellet placement, reagent filling, and buffer mixing, as well as specialized environmental controls, like dry room and DNA/RNA-free assembly areas, are critical capabilities for providing a vertical manufacturing solution for molecular diagnostics.
Successfully commercializing these devices requires additional assembly technologies like ultrasonic welding, precision injection molding (plastics and liquid silicone rubber), various types of specialized adhesives, advanced SMT processes, optical stack assembly, and, most importantly, quality checks.
The entire package of technologies comes together to provide safe, accurate, state-of-the-art tests for the user to take in the comfort of their home. The processes must be executed safely, consistently, and efficiently, enabling an OEM’s innovative technology to be brought to their target market in a device manufactured at an economically sustainable cost of goods sold.
Checklist of an Exceptional Manufacturing Partner
Being exceptional at innovation is a major competitive advantage for OEMs. This is where strategic partnerships come into play.
Do best by being best at what you do, and welcome partnerships that meet the rest of your needs. For example, we see many top-tier OEMs increasing organizational focus on product innovations in molecular detection and therapeutics and leaning on partners to develop, optimize, and deploy commercial solutions for their device portfolio.
Partnering with a provider that checks all these boxes is a winning strategy for device OEMs looking to maintain the critical balance of innovation and cost management required to bring complex, novel technology solutions to market.
Ensure such partners offer:
Verticalized Solutions
Single-site offerings so that manufacturing requirements, including niche capabilities, are aggregated into a single supply source for your business.
End-to-end solutions that:
Simplify supply chain management
Improve quality performance
Increase your ability to scale and
Guide regulatory compliance, from development through market approval.
Global Footprint and Scale
Manufacturing locations in your target market’s backyard.
Strategic site solutions that help to minimize tariff burdens and/or provide low-cost manufacturing locations around the world as well as provide options to improve business continuity.
Broad Industry Experience and Insight
Expertise leveraged across multiple industry sectors:
Specialized capabilities, including surface modification, lyophilization, pellet placement, reagent filling and buffer mixing, dry rooms, and DNA/RNA-free assembly areas.
Broader capabilities, including precision mechanics, photonics, component miniaturization, digitization/data solutions, and advanced molding.
Strategic insights, including comprehensive global supply chain intelligence, predictive product lifecycle management, and sustainability practices.
Case Study: Bringing a Complex Assay to Market
ams OSRAM is a global leader in optical solutions. They had game-changing sensor technologies for digitizing lateral flow tests, but needed a partner versed in healthcare with scalable manufacturing capabilities to help deliver their device concept and technology to market. They also set a high bar for turnaround time. They wanted to do it in less than 12 months.
Explained Filip Frederix, director of ams OSRAM’s digital health segment: “What is currently limiting point-of-care diagnostics is that you don't want to jeopardize the performance of the test with a poor reading. That's also why we wanted to add the electronics and optical components to an existing, established lateral flow test with the ambition to deliver lab-based quality. You need to be able to mass produce it in a cost-effective way.”
Considering the average digital healthcare product takes between 12 and 18 months to develop, the project’s accelerated timeline would be pushing innovation to its limits—or to borrow Filip Frederix’s words, “At ams OSRAM, we try to imagine the impossible!”
Manufacturing Partner Checklist—Addendum
Passion and drive are critical criteria.
No matter how much cross-industry intelligence your manufacturing partner has, or how many locations they operate around the world, or even how deep their capabilities and service offerings may be, the most exceptional manufacturing partnerships are revealed by the level of inspiration they bring to creating solutions that meet your needs, pushing back on the boundaries of what’s possible, and getting the job done.
Reference
1. Ulrich-Peter Rohr, Carmen Binder and Thomas Dieterle et al. The Value of In Vitro Diagnostic Testing in Medical Practice: A Status Report. PLoS ONE. Vol. 11(3). DOI: 10.1371/journal.pone.0149856
About the Author(s)
You May Also Like




