Materials
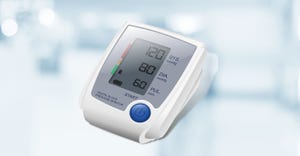
ReadyGo Sampler device
IVD
Porex Partners With UK-based Diagnostics StartupPorex Partners With UK-based Diagnostics Startup
The partnership will scale up production of ReadyGo Diagnostics’ signature device and give it global reach.
Sign up for the QMED & MD+DI Daily newsletter.









.png?width=300&auto=webp&quality=80&disable=upscale)