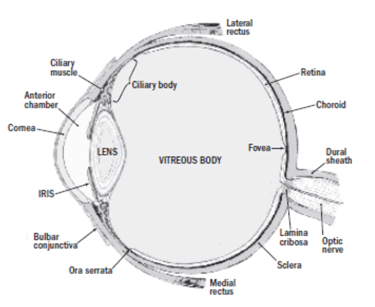
Treatment of various ophthalmic diseases such as viral infections, macular de.g.eneration, and diabetic retinopathy is difficult, in part due to challenges associated with drug delivery to ocular substructures (Figure 1). Therapeutically effective concentrations of a drug are difficult to achieve via topical eye drops (due to poor tissue absorbance, and dilution and runoff due to tearing and blinking) or via systemic administration (due to the blood-retinal barrier).
Treatment of various ophthalmic diseases such as viral infections, macular degeneration, and diabetic retinopathy is difficult, in part due to challenges associated with drug delivery to ocular substructures (Figure 1). Therapeutically effective concentrations of a drug are difficult to achieve via topical eye drops (due to poor tissue absorbance, and dilution and runoff due to tearing and blinking) or via systemic administration (due to the blood-retinal barrier). Furthermore, intravitreal injection can result in complications (e.g., inflammation, infection, increased intraocular pressure, and retinal detachment), especially with repeat administration for chronic conditions. Fortunately, the combination of medical device technologies, polymers and coatings, and drugs is providing a solution for treating various ophthalmic diseases.1,2 Intraocular implantation of drug-device combination products can deliver pharmacologically active compounds to the posterior (back) region of the eye for prolonged periods of time, with minimal systemic exposure and risk for systemic toxicity. A list of current FDA-approved intravitreal implants are provided in Table I. Contact lenses impregnated with a drug may afford greater efficacy for treating conditions of the anterior (front) portion of the eye (such as glaucoma, allergy, dry-eye syndrome), while correcting vision. Advantages for drug-eluting contact lenses, compared to topically administered drug, include ease for patient compliance, sustained drug exposure and the non-invasive manner by which drug delivery occurs.3 Currently, there are no FDA-approved contact lenses designed for drug-delivery.
|
Figure 1. Anatomy of the Human Eye. This image was taken from Smith S, “Basic Ocular Anatomy”, Insight, The Journal of the American Society of Ophthalmic Registered Nurses, Inc., XXXIII(3):19-23, July-Sept, 2008. |
Because of the diversity of drug-device combination products, no single paradigm for testing exists that is appropriate for all products.4 The goal of this article is to provide practical considerations for designing a toxicology/biocompatibility testing program for an ophthalmic drug-device combination product.
General Considerations
The primary goals for toxicology testing of drugs is to characterize dose- and exposure-response relationships, evaluate for potential systemic effects, estimate safety margins, and provide data to guide human dose selection. The extent to which toxicology studies of the drug component of a combination product are conducted is dependent upon whether the drug is already approved for use or is an unapproved new molecular entity (NME).4 A NME requires significantly greater evaluation and data than for an approved drug in which clinical and nonclinical data are already available.5 Familiarity with class effects and/or known safety issues needs to be considered when designing and evaluating toxicology studies for well characterized drugs. When studying a drug-device ophthalmology combination product, it is important to evaluate for both systemic and local (i.e., ocular) effects.6 An initial assessment of local ocular tolerance/irritancy should be considered, depending upon the drug, dose, and/or device components. It is important to understand the pharmacokinetic properties of the drug when used in the combination product. Both local and systemic drug concentrations (and potential drug-metabolites) need to be well characterized when the combination product is implanted or in use. New approaches may be needed to assess effects of localized delivery of the drug, especially when local tissue concentrations are higher than those achieved via systemic administration. For novel device components, safety evaluation of the device alone and in the combination product is warranted. Conversely, safety testing of approved device components would largely focus on the combination product. Pivotal safety tests should include the final design/formulation of the combination product, including the intended means for sterilization.
Test Species and Study Design Considerations
FDA generally requires that toxicity studies be conducted in two species (including one with pigmented eyes).7 Rabbits are commonly used for ocular toxicity studies. Whether or not the drug and/or drug metabolites bind to melanin can be a factor for selecting the strain of rabbit (e.g., New Zealand White albino rabbits versus Dutch Belted pigmented rabbits). Beagle dogs are commonly used as a second species. Although monkeys can be used, they are expensive and challenging to work with. Mice and rats are not recommended for ocular toxicology studies. Studies should include both sexes, with equivalent numbers of animals/sex/group. The experimental design for toxicology studies typically include a control group (vehicle control), and 1 to 3 drug-treated groups, based on dose level. For an implantable device, it is recommended that studies include a surgical sham control, the drug-free device alone as a control, and at least two drug-device groups, at different dose levels. For a drug-eluting contact lens, an untreated control group should be included instead of a surgical sham group.
Evaluation of ocular effects can be performed by gross or macroscopic examination using the Draize scoring scheme, slit-lamp examination of the anterior portion of the eye, and indirect ophthalmoscopic examination of the posterior portion of the eye, including the retina.8,9 Intraocular pressure (tonometry) and corneal thickness (pachymetry) should be measured, and retinal function evaluated using electroretinography.10 At the end of the study, animals should be euthanized and the eyes and adnexa (upper and lower eyelids, lacrimal glands, orbital muscle/tissue) collected for microscopic evaluation. The potential for systemic effects should be evaluated in the ocular toxicity studies, which include clinical observations, physical examinations, body weight and food consumption measurements, and clinical laboratory tests (hematology, clinical chemistries, urinalysis). In addition, blood pressure and electrocardiography are commonly evaluated in dogs and monkeys. The animals should undergo a necropsy with gross organ evaluation, organ weight measurement, and collection of multiple tissues for microscopic evaluation. Blood samples should be collected at various days (time-points) throughout the study to measure for plasma (or serum) drug levels. This will provide information regarding systemic exposure to the drug following implantation of the device. If the device is implanted more than once, then exposure data should be obtained following single and multiple implantations. It is important to know the extent of systemic exposure to the drug and whether it changes following multiple implantations of the device. If the device components are designed to be biodegradable, then evaluation of device resorption and local tissue response should be incorporated into the study. This can be done by collection and microscopic evaluation of the implantation site.
Table I. FDA-Approved Implantable Drug-Device Ophthalmology Combination Products |
Product |
Vitrasert |
Retisert |
Ozurdex |
The kinetics of drug release from the device, and ensuing local tissue concentrations, should be described in animal studies (rabbits as suggested species). This can entail studies designed to collect the device and ocular tissues (e.g., cornea, lens, retina) and fluids (aqueous and vitreous humor) at various times following implantation. The length of the study would be based on the expected release profile of drug, up to a maximum of one year. Depending upon the analytical methods available, the use of radiolabeled drug may be required. Consider whether additional animals are to be included in the toxicology studies to determine ocular tissue drug concentrations at various times.
The device component is expected to be safe and reliable, effective for its intended use, and biocompatible. Good biocompatibility indicates that the device doesn’t produce direct injury to living tissue, adverse inflammatory, immunologic or systemic effects, and does not induce delayed adverse effects. Guidance for medical device biocompatibility testing is well described in the International Standard (ISO) 109933: Biological evaluation of medical devices series of documents (currently comprised of 20 parts). The reader is referred to ISO 10993-1, which serves as an umbrella document for the ISO 10993 series.11
Implantable Devices
Ozurdex (Table I) is a rod-shaped implant comprised of 0.7 mg dexamethasone, USP dispersed in poly (D,L-lactide-co-glycolide) (PLGA) biodegradable polymer matrix and delivered by a single-use applicator device into the posterior segment (vitreous) of the eye.12 Dexamethasone is a corticosteroid that has been used clinically for many years, including ophthalmology indications, to suppress inflammation. Ozurdex is designed to provide sustained release of dexamethasone and was approved as a new drug application (NDA) by the US FDA-CDER in June 2009.
Because dexamethasone is a previously approved drug for both systemic and topical ocular administrations (e.g., Tobradex; 0.1% dexamethasone + 0.3% tobramycin), and PLGA has a long history of safe use in various medical devices, toxicology testing of Ozurdex was primarily focused on the combination product.13,14 Pharmacologic efficacy of Ozurdexwas studied in a rabbit model of corticosteroid-sensitive blood-retinal barrier breakdown, vasculopathy and retinal edema. The potential for dexamethasone to bind to melanin was studied in vitro, and the metabolism of [14C]dexamethasone was studied in human ocular tissues in vitro, and in rabbit and monkey ocular tissues following intravitreal dosing. Distribution of dexamethasone in ocular tissues of rabbits and monkeys was studied following administration of [14C]dexamethasone and Ozurdex. The release kinetics of dexamethasone from Ozurdexinto the vitreous compartment, following single device implantation, were studied in rabbit and monkey studies of up to 6 months duration. Ocular and systemic safety were evaluated in 3 single dose studies (sclerotomy implants) in rabbits, and one-year chronic studies (intravitreal implants) in female New Zealand white rabbits and cynomolgus monkeys. Different doses of Ozurdexwere tested by varying the number of implanted devices (containing 0.7 mg dexamethasone). Treatment groups in the 1-year studies included a surgical sham control, device-alone (placebo), and low (1 implant) and high (2 implants) doses. All animals were treated in the right eye, whereas the left eye remained untreated. The 1-year rabbit study (12/group) included an interim necropsy at 3 months (4/group), repeat administration at three months, and terminal necropsy nine months later. The 1-year monkey study (4/sex/group) included an interim necroposy at 3 months (3/sex/group), repeat administration at three months, and terminal necropsy 9 months later. In addition to microscopic evaluation of ocular tissues in both studies, microscopic evaluation of a comprehensive list of tissues also occurred in the monkey study. Both one-year studies included electroretinography, tonometry, ophthalmic examinations, clinical laboratory tests, and periodic collection of blood for measurement of plasma drug levels after each implantation period. A study was conducted in rabbits to evaluate the safety and performance of the Ozurdex applicator system. Neither genetic toxicology, reproductive and developmental toxicology, nor carcinogenicity studies were conducted for Ozurdex. Dexamethasone has previously been studied in genetic toxicity tests, and produces developmental toxicity in rodents, rabbits and monkeys. Local tolerance of the device was assessed in the various toxicology studies.
|
Figure 2. Retisert Intravitreal Implant. Image obtained Ophthalmology Management. |
Retisert (Table I; Figure 2) is designed to deliver the corticosteroid fluocinolone acetonide (FA) into the posterior region of the eye from a non-resorbable implant (measuring 3 mm x 2 mm x 5 mm).15 Retisert was approved by the US FDA-CDER through the NDA 505(b)(2) process.16 The 505(b)(2) process was applicable because the sponsor submitted some of the toxicology studies of FA from published literature. Retisert is comprised of a tablet containing 0.59 mg fluocinolone acetonide, USP, microcrystalline cellulose, polyvinyl alcohol and magnesium stearate. The device portion is comprised of a silicone elastomer cup with release orifice and polyvinyl alcohol membrane between the tablet and orifice. The cup assembly is attached to a polyvinyl alcohol suture tab with silicone adhesive. Retisertis surgically implanted into the vitreous, and delivers FA for approximately 30 months, after which it can be replaced. As with dexamethasone, FA is a previously approved drug for use as a topical anti-inflammatory agent. The safety of Retisertwas evaluated in toxicology studies in Dutch Belted (pigmented) rabbits and beagle dogs, of 4 weeks (dogs only) and one year duration. These studies included devices that contained varying amounts of active drug. Concentrations of FA were measured in ocular tissues, plasma and urine in the 1-year rabbit study. Genotoxicity studies were conducted on FA (bacterial mutagenicity, mouse lymphoma and murine bone marrow micronucleus assays); carcinogenicity and reproductive toxicity studies were not required because of the low amount of systemic exposure to fluocinolone acetonide following ocular implantation. In contrast to Ozurdex, biocompatibility studies of the Retisert device were conducted as per ISO 10993. These studies included genotoxicity, hemolysis, acute systemic toxicity, dermal sensitization and pyrogenicity tests of extracts prepared from the drug-free device portion.
Contact Lenses
Contact lenses have a long history of safe use, and because they are topically applied, have significantly less potential for adverse effects than implanted devices. Typical biocompatibility studies for contact lenses include the in vitro agar overlay cytotoxicity test,17 acute systemic toxicity study in mice using lens extracts (both polar and non-polar extracts),18,19 an ocular irritation study in rabbits using lens extracts,20 and a 22-day contact lens study in rabbits.21 Biocompatibility testing of a drug-eluting contact lens can incorporate aspects of these studies. It is important to conduct tests on the contact lenses containing the same concentrations of drug as intended to be used clinically. In addition, contact lenses containing higher concentrations of drug can be tested which will provide data for calculating safety margins.
When conducting contact lens studies in rabbits (typically New Zealand white), the lenses should be worn for 7 to 8 hours per day. It is important to observe the animals approximately hourly during the lens wear session to evaluate lens retention. Contact lenses that either fall out or become damaged should be replaced promptly. The lens care solution should be the same as that intended for clinical use. Periodic gross/macroscopic observations of the eyes, during the last hour of lens wear, can be scored according to the Draize scale. The anterior and posterior portions of the eyes should be evaluated pretest and at periodic intervals during the study (after daily lens wear). Potential ocular and systemic toxicity to a drug-eluting contact lens should be evaluated in a chronic rabbit toxicity study. An experimental design could include the following groups: untreated control, drug-free (placebo) contact lens, drug alone (to be administered topically at the high dose level), low dose drug in the contact lens, and high dose drug in the contact lens. This study should be done in both sexes at 6 animals/sex/group (60 total) and involve daily lens wear for 7 to 8 hours per day for 6 months. Both eyes should be similarly treated. In addition to systemic toxicity endpoints previously described, ophthalmic examinations (macroscopic, anterior and posterior portions) should be conducted throughout the study. Electroretinography, tonometry and pachymetry should be performed pretest, and at periodic intervals throughout the study, including termination. Blood samples should be collected throughout the study to monitor for systemic drug exposure. Ocular tissues may be collected from a subset of eyes to evaluate for local drug exposure. A thorough microscopic evaluation of collected eyes and adnexa, and systemic organs should occur.
Summary
In summary, due to the diversity of drug-device combination products being developed for ophthalmology, a “one-size fits all” program does not exist for evaluating safety. Depending upon the drug, device components, implantation/application site and clinical indication, various testing paradigms will exist. However, it is important to evaluate the drug component, the device component, and the combination together as separate entities. An understanding of local and systemic drug exposures arising from device implantation needs to exist. Finally, good science must be the driver behind the testing paradigm.
References
1. Kuno N, Fujii S, “Biodegradable Intraocular Therapies for Retinal Disorders,” Drugs Aging, 27(2):117-134, 2010.
2. Peyman GA, Hosseini K, “Combination Therapies in Ophthalmology: Implications for Intravitreal Delivery,” J Ophth Vis Res, 6(1):36-46, 2011.
3. Ciolino JB, Hoare TR, Iwata NG, et al., “A Drug-Eluting Contact Lens,” Invest Ophthalmol Vis Sci, 50:3346-3352, 2009.
4. Guidance for Industry and FDA Staff: Early Development Considerations for Innovative Combination Products, US FDA, Office of Combination Products, Sept 2006.
5. International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use, “Guidance on Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals, M3(R2),” 11 June 2009.
6. Short BG, “Safety Evaluation of Ocular Drug Delivery Formulations: Techniques and Practical Considerations,” Tox Pathol, 36:49-62, 2008.
7. Weir A, Chambers W, Chen C, et al., “Preclinical Considerations for the Development of Intravitreal Drug Products,” The Toxicologist, 48(1-S):323, Abstract 1524, 1999.
8. Gad SC, Chengelis CP, “Ocular Irritation Testing” in Acute Toxicity Testing, Perspectives and Horizons, Caldwell, NJ, The Telford Press, pp 51-80, 1988.
9. Hackett RB, McDonald TO, “Assessing Ocular Irritation”, in Dermatotoxicology, 5th Edition, Marzulli FN and Maibach HI (eds), Bristol, PA, Taylor & Francis, pp 557-567, 1996.
10. Munger RJ, “Veterinary Ophthalmology in Laboratory Animal Studies,” Vet Ophthal, 5(2):167-175, 2002.
11. ISO 10993-1, Biological evaluation of medical devices, Part 1: Evaluation and testing
12. OZURDEX label, NDA 22-315, Allergan, Inc., 2009.
13. Chambers WA, Division Director Review for NDA 22-315, US FDA-CDER, June 17, 2009.
14. Chen CH, Schmidt W, Pharmacology/Toxicology Review and Evaluation, NDA No. 22-315, Posurdex (dexamethasone biodegradable intravitreal implant) 0.35 mg and 0.7 mg, US FDA-CDER, April 29, 2009.
15. RETISERT label, NDA 21-737, Bausch & Lomb Inc.
16. Chen CH, Yang J, Pharmacology/Toxicology Review and Evaluation, NDA 21-737, RETISERT (fluocinolone acetonide intravitreal implant) 0.59 mg, US FDA-CDER, October 19, 2004.
17. ISO 10993-5, Biological evaluation of medical devices, Part 5: Tests for in vitro cytotoxicity.
18. ISO 10993-11, Biological evaluation of medical devices, Part 11: Tests for systemic toxicity.
19. ISO 10993-12, Biological evaluation of medical devices, Part 12: Sample preparation and reference materials.
20. ISO 10993-10, Biological evaluation of medical devices, Part 10: Tests for irritation and delayed hypersensitivity
21. ISO 9394: Ophthalmic optics – contact lenses and contact lens care products – determination of biocompatibility by ocular study using rabbit eyes.
Alan P. Brown, PhD, DABT, is a senior toxicology consultant at Integrated Nonclinical Development Solutions (INDS; Ann Arbor, MI).
About the Author(s)
You May Also Like