Boston Scientif's subcutaneous implantable cardioverter defibrillator has been considered a scientific milestone in the history of ICDs, but it may not be enough to win commensurate financial rewards.
May 19, 2014
|
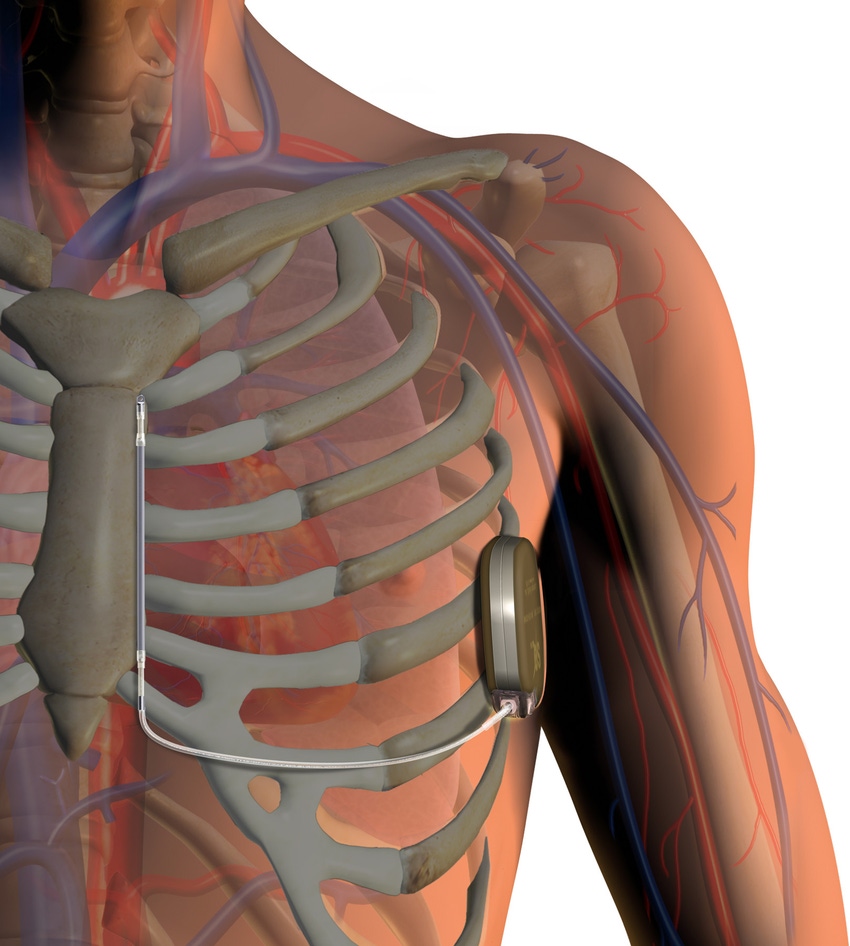
Boston Scientific's subcutaneous implantable defibrillator |
Boston Scientific has high hopes for its breakthrough S-ICD product, the first and only approved subcutaneous implantable cardioverter defibrillator that has no leads in the heart. The product came to the company from the acquisition of Cameron Health.
The company believes that is a disruptive innovation in the ICD market given that ICD leads are widely considered their Achilles heel, and blamed for numerous recalls. Doctors’ interest in the S-ICD has been piqued although Boston Scientific was initially hamstrung by supply issues when the product launched.
Now there is some anecdotal evidence that while interest among physicians is still high, adoption may be less than what Boston Scientific and Wall Street analysts expect.
Glenn Novarro, a medical device analyst with RBC Capital Markets, published a research note Monday where he reports on his conversation with purchasing managers of two of the largest group purchasing organizations (GPOs) in the U.S. They are responsible for a combined $65 billion in sales of devices bought by its hospital member from device vendors. GPOs help to negotiate purchasing contracts between the two sides.
Their take is that doctors are widely using the device in clinical trials but actual penetration of the market will be less than 5% unless the Natick, Massachusetts company can come up with a slimmer design. The S-ICD can measures 78.2 x 65.5 x 15.7 mm in height, width and depth. Compare that to Boston Scientific’s own mini Dynagen Model D020 which measures 67.1 x 52.3 x 9.9 mm.
The current device’s can is lags newer cans by five to seven years in terms of capability, the GPO purchasing managers said.
Here’s more from Novarro’s note:
Current penetration of BSX’s Sub-Q ICD in the GPOs’ member hospitals is ~5-10% as the doctors are trialing the device and trying to determine the optimal patient population. However, the GPOs believe that the penetration rate for the current sub-Q ICD post trialing will be <5%, below our survey results and street thinking. Also, they noted that the sub-Q ICD is being priced ~35-40% above traditional single-chamber ICDs in their hospitals. Given that the sub-Q ICD is not reimbursed at a premium, the GPOs believe that the unfavorable economics will also limit ultimate adoption of the device.
The pricing for the device has once cost Boston Scientific dearly. Hospitals these days want devices that have better clinical outcomes at the same, if not cheaper, price. Given this market reality, along with the fact that reimbursement for S-ICD is not that favorable, Boston Scientific may need to consider lowering the current price while also launching a newer, smaller product able to compete with sleeker ICD cans available in the market.
[Image Credit: Boston Scientific]
-- By Arundhati Parmar, Senior Editor, MD+DI
[email protected]
You May Also Like
.jpg?width=700&auto=webp&quality=80&disable=upscale)


