Edwards Lifesciences, part of the lead pack of companies racing toward a transcatheter mitral valve replacement, announced it has paused its trials for its CardiAQ transcatheter mitral program.
February 2, 2017
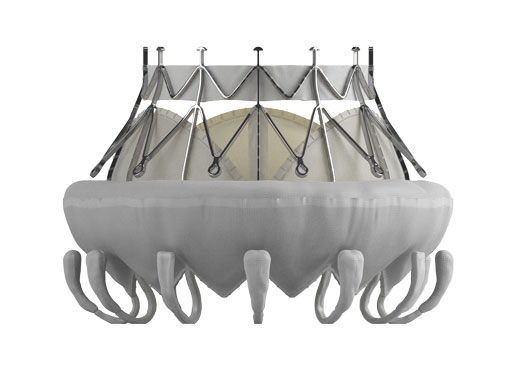
The CardiAQ-Edwards transcatheter mitral valve system is undergoing additional design validation testing.
Edward Lifesciences has announced it is pumping the brakes on trials for its CardiAQ-Edwards transcatheter mitral valve system. The pause is the result of a decision to study a valve feature--not identified in more detail--through additional design validation testing, according to management commentary on the company's latest earnings call.
The move to take on more testing resulted from information gathered during a U.S. early feasibility study of the valve. The voluntary pause halts enrollment in this U.S. study and delays the start of the CE Mark trial. If all goes well in terms of testing, enrollment could get underway again as soon as the second quarter, management said.
According to a Seeking Alpha transcript of the earnings call, Michael Mussallem, Edwards Lifesciences chairman and CEO, explained,
We said that we are engaged in this and we've been going through this process where we tried to very thoughtfully make sure that we learned from all of our clinical experiences. And we saw something that we decided we wanted to--and this is again totally voluntary, go back and take a look at. And so, we're running some internal tests associated with that to make sure that we feel comfortable moving forward. We're doing this in complete cooperation with our clinical investigators. The team continues to be very positive, but it's just a step that we're going to go through."
The company announced back in July 2016 that it was poised to start a CE Mark trial for its Edwards CardiAQ transcatheter mitral valve replacement (TMVR). That trial, called RELIEF, is designed to be a single arm study in patients with functional and degenerative mitral regurgitation at approximately 15 centers in Europe and Canada, using transapical and transseptal delivery approaches.
At Edwards Lifesciences's analyst day in early December, management said that physicians were expressing a preference for a transseptal delivery system. At that time, enrollment in the CE Mark trial was anticipated to start by the end of 2016, though Donald Bobo, Jr., corporate vice president of strategy and corporate development at Edwards, noted that "the regulatory process in Europe has gotten a little more complicated."
Edwards had previously targeted a CE Mark in 2018 for the TMVR system. That timeline may not be off the table, Mussallem pointed out. "We were not saying that the CE Mark is off for 2018, it's still possible for us to get that," he said in response to an analyst question on the earnings call.
Edwards has invested in other mitral valve technologies as well, including with its recent acquisition of Valtech Cardio, an equity investment with option to acquire in Harpoon Medical, and an internal mitral repair system called PASCAL. Although the company has made the mitral valve a priority, the therapy area comes with many hurdles. Commenting on the complexity of the mitral valve, Mussallem said, "I don't know that pausing is that unusual for this sort of an early stage in transformative technology. As I tried to relate earlier, we're on a steep learning curve and to place a valve in the mitral position is a tough task and to do it well . . . We're hopeful that it's positive and that we'll be back rolling again in the second quarter."
[Image courtesy of EDWARDS LIFESCIENCES]
About the Author(s)
You May Also Like


