As FDA considers leadless pacemakers, Boston Scientific is developing a new entry that could be used in coordination with its subcutaneous S-ICD. Here's the latest on that device.
February 22, 2016
As FDA considers leadless pacemakers, Boston Scientific is developing a new entry that could be used in coordination with its subcutaneous S-ICD. Here's the latest on that device.
Marie Thibault
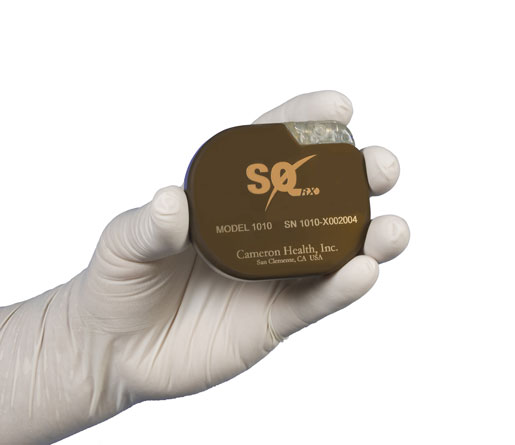
Boston Scientific's leadless pacemaker, in development now, is designed to work as a standalone device or in coordination with the S-ICD, pictured above.
Leadless pacemakers have been a hot field for a couple years now, with two major players—Medtronic and St. Jude Medical—anticipated to be the first to bring the technology to U.S. patients, potentially later this year. But another cardiac company, Boston Scientific is working on what Kenneth Stein, MD, FACC, senior vice president and chief medical officer for the company's cardiac rhythm management group, called a "broader concept of what a leadless pacemaker is and can be."
While Boston Scientific's leadless pacemaker was discussed briefly at the company's investor day in May 2015, Stein gave an update on the device's development at an FDA Circulatory System Devices advisory panel meeting held last week.
The as-yet-unnamed leadless pacemaker is being designed to work alone or in tandem with Boston Scientific's subcutaneous ICD (S-ICD). Terming this a "modular system," Stein explained in an interview with MD+DI that this system would allow "patients who need defibrillators but who also have a need for pacing therapy an alternative that still allows them to avoid transvenous leads."
He added, "You get either one when you need it, but if you develop a need for the other, you can just add the component."
The S-ICD, which received FDA approval in September 2012, is not indicated for use in patients who require pacing therapy. This leadless pacemaker would be able to communicate wirelessly with the S-ICD, Stein said. He said he told the FDA advisory panel that the pacer might be used in any of three situations: for S-ICD patients who later needs pacing therapy, a leadless pacemaker patient who later needs a defibrillator, and lastly, in patients who might need both a defibrillator and pacemaker placed at the same time.
"That does expand the population of patients who could be considered for an S-ICD in the first place, because you don't have to worry about [the need for pacing therapy] happening to them in the future," Stein told MD+DI.
The February 18 FDA advisory panel meeting was held to discuss concerns about adverse events, physician training considerations, and requirements for clinical trials and post-approval studies for leadless pacemakers. The panel discussed clinical trial data on Medtronic's Micra and St. Jude Medical's Nanostim leadless pacemakers, which both have CE Mark.
According to a 24 hour summary of the FDA advisory panel meeting, among the adverse events discussed—device migrations, serious complications at the site of entry (groin), infection, mortality, and pericardial effusion—"the panel felt cardiac perforation rates for LPs (leadless pacemakers) should be consistent with published perforation rates of transvenous pacemakers."
Joanne Wuensch, analyst at BMO Capital Markets, wrote in a February 18 research note, "For the panel, one of the most concerning adverse events was cardiac perforation/pericardial effusion, given combined study results that demonstrated a 50% higher rate than for TV PMs [transvenous pacemakers]."
Stein told MD+DI that with the concerns about both dislodgement and cardiac perforation, the Boston Scientific leadless pacemaker fixation system will use nitinol talons. "What you have to do in terms of design is trade off what gets you the most secure position in the first place so you don't have a risk of the device coming free and dislodging into the general circulation, but on the other hand, minimizing the risk of perforation of the heart," he said.
Stein expects to freeze design of the Boston Scientific device later this year, noting that it is expected to be the smallest sized offering yet, in terms of volume. He added that pre-clinical data showing the viability of the combined system—"that the S-ICD can actually trigger the leadless pacemaker to delivery therapy"—is expected at the American College of Cardiology meeting April 2-4 in Chicago. Clinical trials are expected to start soon after the device design freeze.
The panel meeting seemed to be a positive for the technology. Wuensch wrote, "The panel concluded that leadless pacemakers are a realistic option that could widen the opportunity to include patients that once could not be treated (e.g., high-risk for infection, limited venous access), paving the way for Medtronic’s Micra [estimated FDA approval third quarter of calendar year 2016] and St. Jude Medical’s Nanostim [estimated FDA approval second half of 2016] in the U.S."
Stein said, "It's sort of an unusual thing when [FDA] have a panel that's really not about one company's particular device approval but that's more to look at an entirely new field. I think they get a lot of credit for getting out ahead of the curve on this . . . I thought the panel was very appropriate in trying to balance what we see and what it seemed like they saw as the ultimate promises of this therapy versus trying to think through some of the acute safety concerns and particularly the concern around device perforation."
Get inspired to innovate during Massachusetts Medtech Week—register for BIOMEDevice Boston 2016, April 13-14. |
Marie Thibault is the associate editor at MD+DI. Reach her at [email protected] and on Twitter @medtechmarie.
[Image courtesy of BOSTON SCIENTIFIC CORP.]
About the Author(s)
You May Also Like


