March 12, 2015
Medical device companies have long struggled with understanding design controls and making sure they are implemented correctly to ensure regulatory compliance.
Jon Speer
|
Jon Speer, the founder of greenlight.guru, will give a talk on May 7 at BIOMEDevice Boston titled "Avoiding a Document and Record Scavenger Hunt." |
Design controls have been a challenge to deal with for a long time, despite the fact that FDA design control regulations have been in place since 1996 and the current version of ISO 13485 design and development requirements have been in place since 2003. What's more, FDA and ISO expectations regarding medical device product development are in alignment.
Why is it so hard to deal with design controls? It is not because medical device product development is an unknown science, but it is because capturing the objective evidence to support FDA design controls seems to be more of an art rather than an exact science.
Engineers charged with medical device product development are some of the most brilliant people I have ever met. I'm honored and humbled by the wonders of science and technology that these engineers--true inventors and entrepreneurs--are able to accomplish based on their thoughts, ideas, and ingenuity.
Yet despite this, year after year, complying with design controls is one of the most common areas of deficiency when medical device companies are inspected by the FDA.
Aligning Design Controls with Medical Device Product Development
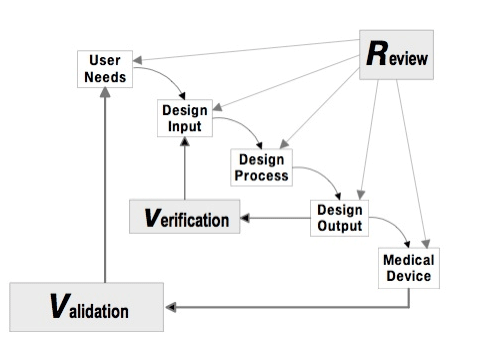
When you read FDA Design Controls as defined in 820.30 or ISO design and development as described in ISO 13485 section 7.3, the steps and criteria are described in a linear fashion.
|
This waterfall diagram shows user needs, followed by design inputs, followed by design outputs, and so on. |
Many medical device companies have adopted more of a phase or "stage-gate" approach to design controls and medical device product development. With this approach, minimum criteria is defined per phase. Once the criteria is met, then the phase can be completed, moving to the next.
These approaches, however, do not seem to fit well with how medical device product development actually works. Medical device product development is never a linear progression. The quest is to always get the product design a step closer to the market.
Design Controls Act as a Framework
Yes, medical device product developers do appreciate the value that design controls brings to the table. Sometimes the value is only perceived because design controls are regulatory requirements. Experienced medical device product developers understand the role that design controls play in the process.
Design controls act as a framework for how to go about designing and developing new medical devices.
Medical device companies interpret FDA design controls regulations and the ISO 13485 design and development requirements into procedures, forms, and templates. These procedures define the framework.
Many articles, case studies, and blog posts have been written on the topic of design controls. There are training courses on design controls taught by medical device industry experts.
To date, the best guide to explain this framework had been the FDA Design Control Guidance for Medical Device Manufacturers.
There is a significant body of knowledge available to describe medical device product development and Design Controls, and all this information is helpful.
Yet the medical device industry still struggles with effective design controls practices.
A Better Way to Navigate Design Controls
Because of the struggles I have experienced with Design Controls throughout my career, I set out to come up with a better way that is clearer to medical device product developers. I wanted to come up with a way that helps provide guidance and direction while helping to ensure best practices that comply with FDA and ISO.
The document titled the Ultimate Guide to Design Controls For Medical Device Startups spells out the necessary background needed about Design Controls from a global regulatory perspective, how to address them, and how they benefit medical device product development.
Divided into 13 sections, the document provides actionable tips for what to do before, during, and after each of the five phases of the medical device product development process and how they relate to Design Controls. This allows for the acceleration of product development while still ensuring regulatory compliance.
Some of the most important highlights of the document are:
The four parts of a quality system to build as you go.
How design controls fit into a broader framework.
How to properly maintain a design history file.
Specific questions to ask to help better understand user needs.
What exactly goes into a design and development plan.
How to effectively and efficiently hold design reviews.
Why design inputs become the roadmap and design outputs become the recipe.
How to prove you designed and built the correct device through V&V testing.
Step by step examples of preparing your regulatory submission.
How to ensure a smooth design transfer.
Hear Speer deliver a talk related to medical device quality at BIOMEDevice Boston, on May 7, 2015. |
Jon Speer is the founder of greenlight.guru and a managing partner at Creo Quality LLC.
About the Author(s)
You May Also Like