February 21, 2017
After a three-week enrollment pause for additional design validation testing, Edwards Lifesciences is ready to get its CardiAQ transcatheter mitral valve replacement (TMVR) program back on track. But the path forward could still be a long one for TMVR in general, according to one medtech analyst.
Amanda Pedersen
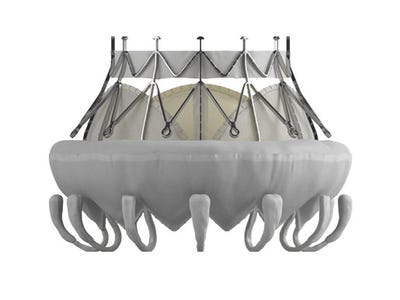
The Edwards CardiaAQ transcatheter mitral valve is ready for clinical trials following the company's voluntary enrollment pause, during which it completed additional design validation testing.
After making a three-week pitstop to complete additional design validation testing, Edwards Lifesciences is getting its groove back with the CardiAQ transcatheter mitral valve. The company noted on its annual Form 10-K, filed with the Securities and Exchange Commission on Friday after the market closed, that it is ready to continue screening patients for enrollment in CardiAQ clinical trials.
Learn about current hot topics and trends of medical device regulation at the ADM Cleveland conference and expo, March 29-30 in Cleveland, OH. |
Most medtech analysts agreed that this is good news for the Irvine, CA-based company, but it does appear that the regulatory track for transcatheter mitral valve replacement (TMVR) devices could be more challenging than the transcatheter aortic valve replacement (TAVR) was.
While the 2003 to 2006 timeframe wasn't a picnic for Edwards and other TAVR players, the path forward in the transcatheter mitral valve market will be "more bumpy and ambiguous than the TAVR experience," according to Canaccord Genuity analyst Jason Mills.
It's still unclear what the specific concern was regarding the CardiAQ valve's design, as Michael Mussallem, chairman and CEO at Edwards, did not go into a lot of detail about it during the company's fourth quarter earnings call. He simply explained at the time that the company had noticed something that it wanted to "go back and take a look at" before proceeding with the trials. The pause halted enrollment in the company's U.S. early feasibility trial and delayed the start of its CE mark study.
Investors reacted favorably to the news that the CardiAQ clinical programs would resume. Edward's stock (NYSE: EW) went up 3.26% ($2.93) to close at $92.67 Tuesday.
Larry Biegelsen, a medical device analyst for Wells Fargo Securities, said the enrollment pause highlights "the potential fits and starts that can occur with any pipeline product in the transcatheter space." He pointed out that the company's first generation Sapien program for TAVR also suffered setbacks before eventually succeeding.
With 11 significant pipeline opportunities in development, Biegelsen said Edwards is in a position to achieve long-term growth even if only one or two of these products in each area are successful.
Long Road Ahead For TMVR
Mills recommended in his report that investors should remain focused on the aortic side of the structural heart field for the near term, as the transcatheter repair and replacement treatment paradigm for the mitral valve will "need at least a few more years in the oven before we know whether we have the right ingredients in the cake."
While it would seem like TAVR is the logical comparator for TMVR, Mills said key opinion leaders have told him that is not actually the case. "TAVR market development and adoption has been one of the most successful medical device stories ever," the analyst said. But he said mitral will require more patience based on the anatomical complexity, heterogeneity of the disease, and undeveloped access to the appropriate care pathways.
On the bright side, Mills and other medtech analysts continue to expect the market opportunity for transcatheter mitral valve platforms will be huge, and roughly three to four times greater on paper than the TAVR opportunity. That should be enough to get investors' attention, Mills said, "especially if we're right and the TAVR market levels off at about $6 billion to $7 billion globally in a decade or so."
Add in the tricuspid opportunity at an estimated U.S. prevalence of 1.5 million, without less than 1% current treatment penetration, Mills said, "and the market potential expands by another few billion at least."
The analyst noted that he has no doubt, based on his research, that clinicians are increasingly and impatiently demanding better solutions for mitral and tricuspid patients. Mills attributed the escalation in demand from clinicians to the success of transcatheter aortic valve procedures, which "at the very least has proven percutaneous approaches to structural heart disease are feasible, if not superior, to surgery."
That has certainly been the case on the aortic side, Mills said, adding that "time will tell" if it is also true for mitral valve procedures.
Amanda Pedersen is Qmed's news editor. Contact her at [email protected].
[Image credit: Edwards Lifesciences]
About the Author(s)
You May Also Like


