January 12, 2016
The major LVAD maker no longer has a timeline for its next-generation MVAD system.
Nancy Crotti
Updated January 13, 2016
|
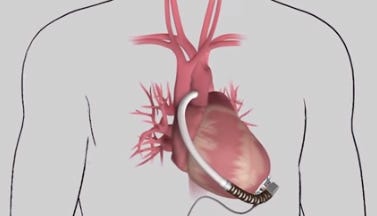
HeartWare's MVAD is one-third the size of its HVAD pump. (Image courtesy of HeartWare) |
Shares of LVAD maker HeartWare International plunged 35% Tuesday, closing at $26.50 per share, on news of lower earnings and uncertainty about the future of its new MVAD system.
About 3 million shares changed hands soon after the company's announcement that profits had fallen to $68 million for the fourth quarter of 2015, down from $73.2 million for the same period one year ago.
The next day, St. Jude Medical--the owner of HeartWare's chief competitor Thoratec--announced better-than-expected fourth-quarter sales for Thoratec.
On top of the negative earnings news, HeartWare President and CEO Doug Godshall said the company could not set a date for a new clinical trial of its next-generation MVAD system. The Framingham, MA-based company halted a trial for the CE mark in October. Godshall said it found "certain algorithms, which appear to increase the potential" for blood clots in the pump, a potentially deadly condition associated with ventricular pumps in general.
"While the initial MVAD clinical experience has not fully met our expectations, we have made meaningful progress in our investigation and have identified some software algorithms that we plan to adjust to improve pump performance," Godshall added. "As the MVAD System is performing well on most fronts, we remain optimistic that an improved MVAD System will emerge from this additional evaluation."
Investors dumped HeartWare stock in October as well, on the heels of competitor St. Jude Medical winning the CE Mark for the HeartMate 3 Left Ventricular Assist System Device (LVAD). St. Jude had just acquired the HeartMate 3 through its $3.3 billion purchase of Thoratec.
St. Jude reported on Wednesday that Thoratec saw sales of $143 million during the fourth quarter of 2015, beating previous guidance from the company. Thoratec's sales were up 15% year-over-year minus currency-related effects.
Following Tuesday's news, thestreet.com gave HeartWare a "D" rating, urging investors to sell due to "weaknesses" in multiple areas, including deteriorating net income, disappointing return on equity, generally high debt management risk, generally disappointing historical performance in the stock itself and feeble growth in its earnings per share."
FDA warned in August of an increased rate of blood clots in Thoratec's HeartMate II and a high rate of stroke among people using the HeartWare HVAD. There have also been bleeding complications.
Learn more about cutting-edge medical devices at MD&M West, February 9-11 at the Anaheim Convention Center in Anaheim, CA. |
Nancy Crotti is a contributor to Qmed and MPMN.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like