June 30, 2016
Thousands of HVADs could have faulty battery cells.
Nancy Crotti
|
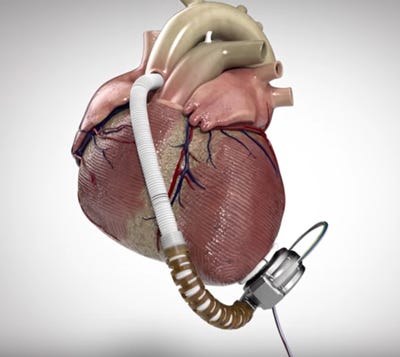
HeartWare's HVAD (Image courtesy of HeartWare) |
HeartWare International is recalling more than 18,000 batteries for ventricular assist devices it sold between 2013 and 2015, the company and FDA recently announced.
The batteries may lose power prematurely due to faulty cells, according to a notice from FDA, which has designated the recall Class I. The Framingham, MA-based company's HVAD system includes a pump implanted in the space around the heart and a controller that regulates the pump's speed and function.
The news comes on the heels of Medtronic's announcement that it intends to purchase HeartWare for $1.1 billion. HeartWare notified customers of the recall in January, but FDA just announced it Thursday. A Medtronic spokesperson says the medical device giant was aware of the recall when it offered to buy HeartWare.
The deal, already approved by both companies' boards of directors, expands Medtronic's reach to more heart-failure patients. It also puts Medtronic on more even footing in the minimally invasive ventricular assist device market with St. Jude Medical, which acquired LVAD-maker Thoratec and its HeartMate III technology for $3.3 billion in 2015. HeartMate III won the CE Mark in October, and won FDA approval earlier last year to enlarge the pivotal trialfor the next-generation device.
HeartWare has "multiple technologies" in development for even less-invasive devices for patients with end-stage heart failure, according to Medtronic. The medtech giant estimates that the global VAD market at approximately $800 million, and predicts it will grow by tens of millions of dollars in next couple of years.
Indeed, both the Medtronic and St. Jude Medical acquisitions of LVAD companies could expand the technology's reach, and perhaps boost innovation, Qmed's sister media outlet MD+Di reports.
The latest recall, one of a series for HeartWare, affects devices used in heart-transplant candidates who are at risk of death from end-stage left ventricular heart failure. If the HVAD system is not connected to an additional power source shortly after the low-battery alarm sounds, the pump will stop working and the patient could die, according to FDA.
No deaths have been directly attributed to the batteries' failure, but several adverse events have been reported.
This recall includes batteries used on HeartWare HVADs carrying the serial numbers BAT000001 to BAT199999 and model number 1650, manufactured from May 19, 2013 to July 1, 2015 and distributed from May 21, 2013 to July 31, 2015. A total of 18,631 units were recalled nationwide, FDA noted.
VADs have been plagued with problems and competition in the field is fierce. HeartWare's stock plunged in January on news of lower earnings and uncertainty over whether the company would set a date for a new clinical trial of its less invasive,next-generation MVAD system. The company halted a trial for the CE mark in October due to "certain algorithms, which appear to increase the potential" for blood clots in the pump, a potentially deadly condition associated with ventricular pumps in general.
Since FDA approval of the devices, there has been an increased rate of pump thrombosis (blood clots inside the pump) among people using Thoratec's HeartMateII, and a high rate of stroke among people using the HeartWare HVAD, FDA says. There have also been bleeding complications.
HeartMate II received FDA approval as a bridge-to-transplant device in 2008 and a destination therapy device in 2010. HeartWare's HVAD received approval as a bridge device in 2012.
Nancy Crotti is a contributor to Qmed.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like