June 16, 2017
Neovasc said it will appeal a ruling that partially favored Edwards in a European patent dispute.
Amanda Pedersen
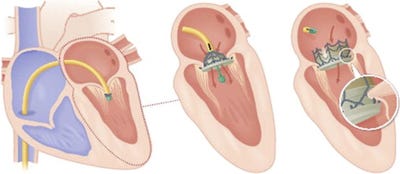
The CardiAQ device is threaded into the heart from below.
Vancouver-based Neovasc said it will appeal a German court ruling that partially favored Irvine, CA-based Edwards Lifesciences Corp. in a European patent dispute.
The District Court in Munich found that CardiAQ Valve Technologies Inc., which is now part of Edwards, contributed to the invention of Neovasc's Tiara mitral valve replacement (TMVR) technology. The court awarded Edwards co-entitlement rights to the patent in question, but no monetary awards.
Neovasc is already appealing a 2016 U.S. district court decision, which also declared CardiAQ co-inventor on a U.S. patent application for the same technology. The company said it expects to argue the case in August, and should have a ruling on the matter before the end of the year.
Pending the outcome of the U.S. case, Neovasc said it will "vigorously defend its position" that the German case is without merit, and will explore its options to appeal the recent decision.
The battle between CardiAQ and Neovasc was born when CardiaAQ accused Neovasc, its former service provider, of breaching a non-disclosure agreement and misappropriating trade secrets. CardiAQ co-founders Arshad Quadri, MD, and J. Brent Ratz hired Neovasc in 2009 to provide tissue processing and valve assembly services. But CardiAQ ended up suing Neovasc in 2014 after discovering a late 2011 Neovasc patent publication. Neovasc had been working on its own TMVR program, without disclosing the program to CardiAQ, according to that lawsuit.
Edwards paid $400 million for CardiAQ in 2015. TMVR represents a huge market opportunity for medtech companies, with most analysts predicting that the field will grow to $6 billion or so over the next decade. Medtronic and Abbott Laboratories have also spent a lot of cash in recent years to buy smaller companies with TMVR technology in the pipeline.
Still, the regulatory track for TMVR has proved to be a bit more challenging for companies than the approval pathway for transcatheter aortic valve replacement was. Edwards had to pause its clinical trial program for the CardiAQ valve earlier this year, but now appears to be back on track with the technology.
Amanda Pedersen is Qmed's news editor. Contact her at [email protected].
[Image credit: Edwards Lifesciences]
About the Author(s)
You May Also Like


