October 15, 2015
Spinal implant firm NuVasive recently received 510(k) clearance for a spinal implant for cervical corpectomy, marking the first time that the agency had approved or cleared a medical device for this intended use. One of the predicate devices used to grant the clearance was at the heart of a $13.5 million fraud settlement NuVasive made with the DOJ.
Qmed Staff
|
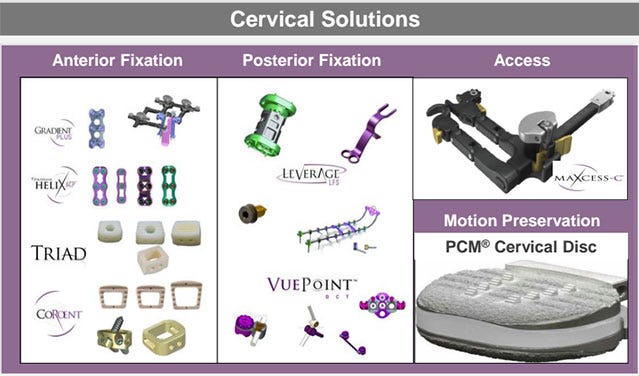
A 2012 NuVasive slide presentation shows the X-Core Mini Cervical Expandable VBR system (in green) as part of the company's portfolio of cervical solutions. |
NuVasive (San Diego) recently announced that it was the first company to receive FDA clearance for a unique neck implant known as a cervical corpectomy cage--a device used to replace a cervical vertebrae and adjacent discs in the treatment of multilevel cervical spondylototic myelopathy (CSM), the most common spinal cord disorder in the world. NuVasive's announcement describes the product as a "first-ever FDA 510(k) clearance" for this product type.
NuVasive was able to convince FDA in 99 days that the novel product X-Core Mini Cervical Expandable VBR system (K151651) was substantially equivalent to two predicate devices: the NuVasive X-Core Expandable VBR System (K142205) and the NuVasive CoRoent system (K081611). While the X-Core Mini is indicated for use in the cervical spine, both of its predicates were designed and indicated for use in the thoracic and lumbar spine (T1 to L5). NuVasive's 510(k) application for the X-Core Mini was received by FDA on June 18, 2015 and cleared on September 25.
The biomechanical behavior of the cervical spine is unique as it lacks the support of the rib cage found in the kyphotic thoracic spine, and is markedly smaller than the much larger vertebrae found in the lumbar spine which carries the greatest load in the body. Any injury to the cervical spine can be catastrophic causing paralysis or weakness in both arms and legs (quadriplegia), respiratory issues, bowel, bladder, and sexual dysfunction. This area of the spinal cord controls signals to the back of the head, neck and shoulders, arms and hands, and diaphragm. Since the neck region is so flexible it is difficult to stabilize cervical spinal cord injuries.
According to FDA, a previously cleared or approved device that has undergone modifications that changes the intended use or that could significantly affect safety and effectiveness must obtain an approved application for premarket approval (PMA), 510(k), or an exemption. Prior to FDA clearing the X-Core Mini for cervical corpectomy, there was no legally marketed cervical corpectomy device. Typically, a high-risk "first of its kind" device would be subject to a de novo classification, then a likely investigational device exemption (IDE) for human testing so that safety and effectiveness can be verified. The X-Core Mini appears to have dodged the de novo and IDE process, which would have cost NuVasive millions to conduct clinical trials.
The X-Core Mini device, though just cleared for cervical corpectomy by FDA, has been marketed outside of the United States in sizes designed for cervical indications under the same trade name X-Core Mini since at least 2012. In Brazil, Australia, the identical device has been sold for cervical corpectomy. The product was also included in a slide deck accompanying NuVasive's 2012 Analyst Meeting where it is listed and shown among the company's "cervical solutions."
The second predicate device, CoRoent VBR, was named in a recent DOJ lawsuit, United States ex rel. Kevin Ryan v. NuVasive, Inc., which alleged that NuVasive promoted the corpectomy device for surgical uses that weren't approved by the FDA. DOJ alleged that this behavior resulted in widespread Medicare fraud. NuVasive agreed to pay $13.5 million to settle the allegations out of court. Following the announcement of the deal, NuVasive's stock dropped 13% in a single day earlier this year.
On October 15, Kentucky attorney general Jack Conway announced that the state has joined the ongoing lawsuit tied to the CoRoent. "Reckless promotion of medical devices resulting in false claims to state and national healthcare programs is unacceptable," said Attorney General Jack Conway in a statement. "Companies that exploit Kentucky taxpayers through deceptive marketing practices and illegal kickbacks will face the consequences."
In 2011, Medtronic won a court case against NuVasive, which alleged that its CoRoent XL among other products infringed on Medtronic's patents. The jury awarded Medtronic $101.2 million in damages in that case, but the award was overturned on appeal.
In 2013, NuVasive also received a warning letter for selling adulterated and misbranded products.
The X-Core device was cleared by FDA based on its similarity or "substantial equivalence" to the NuVasive's Expandable Lumbar Interbody System and four other devices, including the Synthes Spine Synex Spacer System, which was subject to a Class I recall in 2009. FDA's MAUDE database reveals seven adverse events connected to the X-Core device, largely from device "collapse" or "loss of distraction (height)." The Synex was recalled after just six adverse events reporting similar device failures.
The new X-Core Mini device is unlike its two predicates in that it is designed to specifically fit and conform to the size and shape of the endplates and spinal curvature found only in the cervical spine C3-C7. In the past, this procedure called for replacing the vertebra with either hip or fibula bone graft and often screwing a metal plate to the graft to anchor it in place so that it can fuse with the surrounding vertebrae. The X-Core Mini was designed to give orthopedic surgeons the option of using a distractable cage that can be intraoperatively filled with grafting material, rather than pre-loading it before implantation.
When asked for a comment, FDA press officer Deborah Kotz referred to subheads F and G of the 510(k) granted for the device. The first subhead reports that the X-Core Mini "was shown to be substantially equivalent based on clinical data and have the same technological characteristics to its predicate devices through comparison in areas including design, intended use, performance, material composition, and function." Section G explains that "clinical performance data on the subject device, as well as clinical literature review and retrospective clinical data analysis was performed to support the use of the subject device for expanded indications for use."
The 510(k) clearance for the X-Core Mini, which includes the requirement that the device be used with an appropriate supplemental fixation device such as the company's NuVasive's Archon-R plate, is unique in that it may represent a unique interpretation of FDA's 510(k) substantial equivalence doctrine, which requires the device in question to have "the same intended use as the predicate device," according to the FDA website. As mentioned before, no device had yet been approved or cleared for cervical corpectomy, and its predicate devices were indicated for use in other parts of the spine.
FDA recently established the new product code "PLR" for cervical corpectomy. Before that decision, there was no legally marketed devices for this intended use. Unlike product code "MQP" designated for non-cervical corpectomy devices, there are no specifically recognized ASTM or ISO conformance standards for cervical corpectomy. So it raises the question, how did FDA make a determination of "substantial equivalence" with no clinical or biomechanical standards to follow?
FDA's Division of Orthopedic Devices (DOD) headed by Mark Melkerson M.S., where K151651 was reviewed, has a long history of clearing devices that have subsequently been involved in major U.S. product liability litigation. Recent examples include litigation surrounding metal-on-metal hip implants.
At the time of writing, NuVasive had not responded for a request for comment.
Learn more about cutting-edge medical devices at Minnesota Medtech Week, November 4-5 in Minneapolis. |
Like what you're reading? Subscribe to our daily e-newsletter.
Note: An earlier version of this article incorrectly stated that the device was cleared by FDA in less than one month. The submission was received by FDA on June 18, 2015 and cleared on September 25, 2015. The original date in the article mentioned as the submission date (8/27/2015) referred to the date FDA received the revised 510(k) summary.
About the Author(s)
You May Also Like