LumiSystem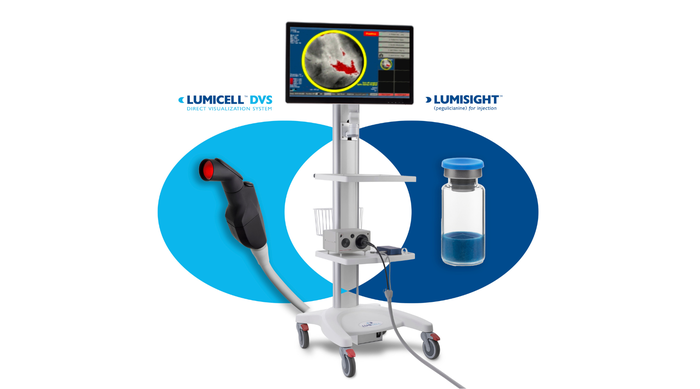
Medical Device Regulations
Breast Cancer Detection Drug-Device System Nabs FDA ApprovalBreast Cancer Detection Drug-Device System Nabs FDA Approval
The LumiSystem enables surgeons to scan the breast cavity post-lumpectomy in real time, detect, and resect residual cancer that otherwise could have been missed.
Sign up for the QMED & MD+DI Daily newsletter.





















.png?width=300&auto=webp&quality=80&disable=upscale)


.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)


.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)


