August 5, 2015
The agency's safety communication reports serious adverse events associated with both Thoratec and HeartWare devices.
|
HeartMate II, as shown on Thoratec's website |
Chris Newmarker
Major LVAD makers Thoratec and HeartWare were dealt a setback Wednesday when FDA issues a safety communication reporting problems with the two companies' U.S.-approved devices.
Since FDA approval of the devices, there has been an increased rate of pump thrombosis (blood clots inside the pump) among people using Thoratec's HeartMate II, and a high rate of stroke among people using the HeartWare HVAD, FDA says. There have also been bleeding complications.
HeartMate II received FDA approval as a bridge-to-transplant device in 2008 and a destination therapy device in 2010. HeartWare's HVAD received approval as a bridge device in 2012.
FDA officials still think the benefits of LVADs outweigh the risks when it comes to saving the lives of people with advanced left ventricular heart failure. But FDA also wants health care providers and their patients to be aware of the risks when considering use of the devices.
Thoratec and HeartWare spokespeople could not be immediately reached for comment. A spokesperson at St. Jude Medical, which is in the process of acquiring Thoratec for $3.4 billion, declined to comment.
The news comes only a week after a Mayo Clinic study showed that LVADs succeeded in treating patients with potentially deadly restrictive cardiomyopathy (RCM), suggesting more uses for LVADs.
Thoratec's HeartMate II is touted as the most widely used and extensively studied left ventricular assist device. Earlier this year, the Pleasanton, CA-based company received FDA approval to enlarge its pivotal trial for its much-anticipated, next-generation HeartMate III.
|
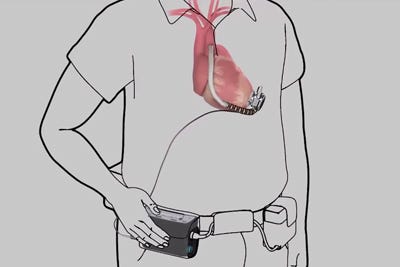
HeartWare's driveline cable, shown in this illustration from a company video, connects the implanted pump with an externally worn controller. |
Framingham, MA-based HeartWare has meanwhile started patient enrollment in its MVAD System CE Mark trial. The MVAD is one-third the size of the HVAD Pump.
"And we were just down there--I was just down there [July 27] with the FDA--and they are incredibly supportive and really trying to figure out how to help us get through their requests as quickly as we can and looking for ways to expedite the process which is, frankly, quite refreshing relative to what we have experienced historically. And so we're really encouraged by the level of support we're getting from the device evaluation branch is great," HeartWare CEO Doug Godshall said in a Friday earnings call with analysts, transcribed by Seeking Alpha.
FDA, though, is now warning of serious and potentially deadly complications when it comes to the Thoratec HeartMate II and the HeartWare HVAD:
With the HeartMate II, scientific studies have found a pump thrombosis rate as high as 8.4% of implanted devices at three months and 6% at six months. That's higher than the 1.6% rate found after one year during the clinical trial to support bridge-to-transplant approval in 2008, and the 3.8% rate found after two years in the clinical trial for destination therapy approval in 2010.
During a clinical trial to evaluate the HeartWare HVAD as a destination therapy, 28.7% of HVAD patients experienced one or more strokes over two years, versus 12.1% among patients implanted with the HeartMate II.
FDA has also become aware of bleeding complications related to both the Thoratec and HeartWare devices. The cause of the bleeding complications--mentioned in adverse event reports and a variety of other sources--is not fully understood and likely due to many different factors, according to FDA.
Both companies are presently conducting studies to better understand the problems that FDA has identified.
LVADs have had their share of recalls in recent years.
Thoratec last year issued an urgent advisory over four patient deaths related to its HeartMate II device. Five other patients lost consciousness or experienced reduced blood flow when attempting to use backup system controllers for the pump. The deaths and serious injuries resulted not from device failure, but rather patients and caregivers who were unable to understand instructions.
HeartWare has had its own string of run-ins with FDA over its heart pump, manufactured in Miami Lakes, FL.
The company received 27 complaints between February 2010 and November 2013, including reports of two deaths and four serious injuries, related to ventricular assist device system, but failed to verify or validate the effectiveness of corrective actions, according to a June 2014 FDA warning letter.
FDA also issued a Class I designation over a voluntary recall involving the system in April 2014, saying that a faulty driveline connector could cause the heart pump to temporarily stop. And HeartWare had another Class I-level recall this year after one serious injury and 33 complaints of malfunction related to worn down power-supply connector ports.
Refresh your medical device industry knowledge at MEDevice San Diego, September 1-2, 2015. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like