Ivenix Infusion Pump LVP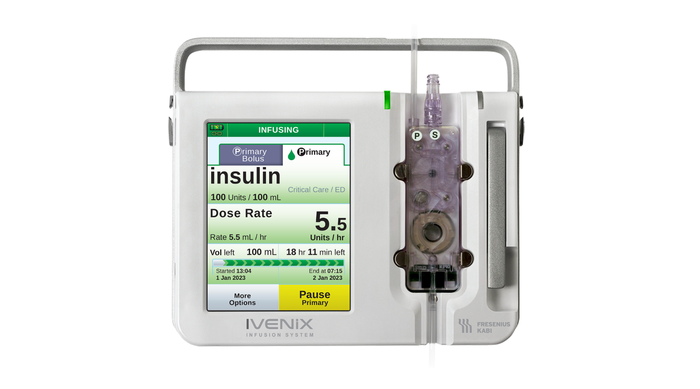
Regulatory & Quality
Fresenius Kabi USA Infusion Pump Software Recall Declared Class IFresenius Kabi USA Infusion Pump Software Recall Declared Class I
The company is urging customers to update the device software in order to mitigate noted software anomalies.
Sign up for the QMED & MD+DI Daily newsletter.




















.png?width=300&auto=webp&quality=80&disable=upscale)


.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)


.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)