May 19, 2015
FDA advisory panel recommends costly sterilization before reuse amid deadly superbug outbreaks.
Why Duodenoscope-related Infections Are a Big DealFDA's reaction to duodenoscope-related infections could ultimately impact makers of nearly all medical devices, says Michael Drues, PhD, president of Vascular Sciences (Boston). |
By Nancy Crotti
An advisory panel to the Food and Drug Administration has recommended drastically improving cleaning techniques for duodenoscopes linked to superbug outbreaks at hospitals in Seattle, Los Angeles, and outside Chicago.
During a two-day hearing, several panel members called for hospitals to sterilize the scopes before reuse, a costly process that uses ethylene oxide gas, which could be toxic to workers.
FDA is not bound by its panels' advice, and can only recommend procedures to hospitals. The agency usually follows the recommendations of its advisory panels, according to reports in the Los Angeles Times and the Wall Street Journal.
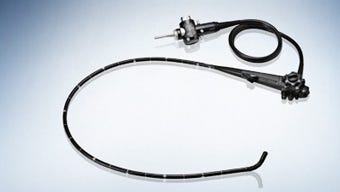
Sold in the United States by manufacturers including Fujifilm, Olympus, and Pentax, duodenoscopes are threadeddown through the digestive tract and into the small intestine. They provide the least invasive way of draining fluids from pancreatic and biliary ducts blocked by cancerous tumors, gallstones, or other conditions. The duodenoscopes' movable "elevator" mechanism, located at the tip, improves efficiency and effectiveness but has proven challenging to disinfect, according to FDA.
The Journal story called the panel's decision a "high-profile rebuke" of both FDA and Olympus, which dominates the U.S. duodenoscope market. Federal officials have investigated nine superbug outbreaks related to duodenoscope use, the newspaper said.
None of the three major duodenoscope manufacturers sent representatives to the two-day hearing held in Silver Spring, MD, the Journal reported. An Olympus representative recently told Qmed that the company "taking this matter extremely seriously."
Michael Drues, PhD, president of Vascular Sciences (Boston), said recently that this problem is not unique or limited to products like duodenoscopes. Separately, some panel members concurred, pointing out that bronchoscopes, colonoscopies and similar reusable devices may also transfer pathogens.
UCLA has begun sterilizing duodenoscopes with ethylene oxide, but the gas is so toxic that doctors must wait longer before reusing the devices, the L.A. Times reported. That has led to demand for more duodenoscopes, the newspaper said. FDA estimates that about 600,000 procedures per year involve duodenoscopes.
At least seven patients were infected and two died from the drug-resistant bacterial strain carbapenem-resistant Enterobacteriaceae (CRE) at UCLA's Ronald Reagan Medical Center from October to January 2014. The hospital believes that 179 patients may have been exposed to CRE from a contaminated, reprocessed Olympus duodenoscope.
At Virginia Mason Medical Center in Seattle, at least 39 people developed multidrug-resistant infections following endoscopic retrograde cholangiopancreatography procedures (ERCP) between 2012 and early 2014. The staff recently automated the cleaning procedures for the scopes, the L.A. Times reported.
At least 18 affected patients at Virginia Mason have died, although the Seattle Times reported that it is not clear whether the infections from the scopes played any role in their deaths.
FDA issued a new guidance document in March for reprocessing reusable medical devices.
Refresh your medical device industry knowledge at MD&M East in New York City, June 9-11, 2015. |
Nancy Crotti is a contributor to Qmed and MPMN.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like