June 27, 2014
The ReWalk Personal System has become the first FDA-cleared wearable, motorized device to aid paraplegics--another first when it comes to what exoskeleton technology is enabling in the medical device field. The device was cleared via the de novo 510(k) classification process.
|
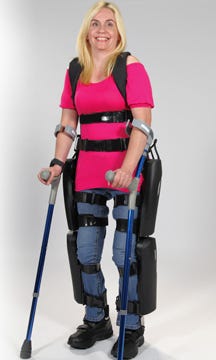
ReWalk Personal System |
Developed by Argo Medical Technologies (Marlborough, MA), the ReWalk had already been on the market in Europe since 2012. The company has distribution partners in Great Britain, France, Italy, Austria, Japan, and Turkey.
ReWalk includes a fitted, metal brace that supports the legs and part of the upper body; motors that provide movement at the hips, knees, and ankles; a tilt sensor; and a backpack containing a computer and power supply, according to the FDA. The FDA adds: "Crutches provide the user with additional stability when walking, standing, and rising up from a chair. Using a wireless remote control worn on the wrist, the user commands ReWalk to stand up, sit down or walk."
According to FDA, the device is intended for patients paralyzed from spinal cord injuries ranging from the seventh thoracic vertebra to the fifth lumbar vertebra when it is used in conjunction with a trained caregiver. In rehabilitation clinics, it can be used for patients with spinal cord injuries ranging from the fourth thoracic vertebra to the sixth thoracic vertebra.
The ReWalk retails for approximately $70,000 in Europe and will likely have a similar price tag in the United States.
The device was invented by an Israeli researcher named Amit Goffer who has been paralyzed since 1997.
"This revolutionary product will have an immediate, life-changing impact on individuals with spinal cord injuries," Larry Jasinski, CEO of ReWalk Robotics, said in a news release. "For the first time individuals with paraplegia will be able to take home this exoskeleton technology, use it every day and maximize on the physiological and psychological benefits we have observed in clinical trials."
In 2012, a British woman named Claire Lomas made history by using the ReWalk to compete the London Marathon.
Refresh your medical device industry knowledge at MEDevice San Diego, September 10-11, 2014. |
ReWalk is not alone in the development of exoskeleton technology to aid the paralyzed.
An exoskeleton developed by Ekso Bionics (Richmond, CA) evolved from a platform known as HULC, an acronym for "human load universal carrier." Ekso has licensed the hydraulic-powered anthropomorphic exoskeleton technology to Lockheed Martin.
To create the medical version of the device, Ekso developers took the military-grade HULC, and modified the actuation technology while adding new sensing and software functionality. After a medical version of the exoskeleton had been created, its developers believed that, at some point in the future, it could replace wheelchairs for many patients suffering from mobility disorders. Now, however, the company's is focusing on how to expand the scope of the technology to help those who are suffering from any amount of lower-extremity weakness.
Traditionally, robotics technology has been confined to structured environments where it can be used to perform repetitive tasks. Bringing robotics, tethered to humans, out to the unstructured environment found in the everyday world has required parallel advances in mechanical engineering, electrical engineering, and software.
Even more dazzling innovations could come as researchers figure out less intrusive mind-computer interfaces. It requires extremely sophisticated readings and translations of electroencephalography (EEG) signals, but someday, a paralyzed person might be able to control an exoskeleton with an EEG cap.
Related article
Brain Chip Breakthrough Enables Paralyzed Man to Move Hand
Chris Newmarker is senior editor of MPMN and Qmed. Follow him on Twitter at @newmarker. Brian Buntz is the editor-in-chief of MPMN and Qmed. Follow him on Twitter at @brian_buntz.
About the Author(s)
You May Also Like