Typically, medical device companies focus on product innovation, regulatory compliance, and effective sales and marketing. These strategies, combined with positive demographic trends of aging populations in the developed world and increasingly good healthcare in populous developing countries, have led most companies to enjoy strong volume growth. But what are the most successful companies doing to capitalize on this opportunity?
|
|
Julie Fraser | Jeff Peabody |
If they are smart, they are focusing their efforts to meet the challenges that rapid growth creates in manufacturing operations. Not only is the plant expected to push through higher volumes, but also higher mix (i.e., more products with more versions, models, or options). This rapid growth can cause a real strain on the production operation. The complexity of ramping up volume and handling a mix of new and established product is intense, particularly since the regulatory requirements in each new market and geography will vary.
Download the Cambashi Inc. and UBM Canon study. |
The requirements for production, quality, and regulatory departments can increase dramatically, as follows:
Manufacturing must manage more standard operating procedures (SOPs), and cope with new product differences from current ones.
Engineering must be able to quickly develop processes to make new products efficiently and at high quality.
Quality must manage specifications, risk analysis, test and quality assurance protocols for many more products.
Regulatory must develop and sign off on documentation for more different products and a higher volume of them.
For companies managing their production environment in a manual or partially automated way, each of these are nearly insurmountable challenges. All of these efforts need to be coordinated, with each group sharing and using relevant information in a timely manner. Many medical device companies do not have a modern, flexible manufacturing execution system (MES) to handle these requirements across their enterprise.
|
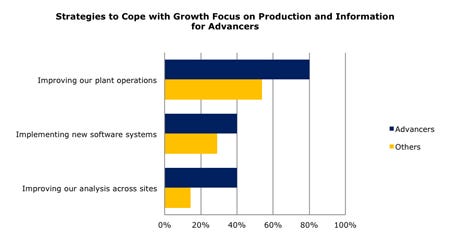
Figure 1: 80% of Advancers are focused on improving plant operations; they are also more likely to be using information wisely than others. It appears to be working: these companies improved profits and grew volumes at the same time. Source: Beyond Tradeoffs in Medical Device Manufacturing: Balancing Innovation, Quality and Compliance While Improving Profit © 2012 Cambashi Inc. and UBM Canon |
As a result, while most companies are growing volume, only a fraction are increasing their profitability at the same time. The quarter of companies in this market that are improving both profits and volume—what we’ll call Advancers—are really finding they must focus on improving their plant operations. As Figure 1 shows, this is the best strategy for coping with growth for four out of five of those Advancers. Another 40% indicate that implementing new software systems is key and 40% indicated improving analysis across sites was critical. The strategies are not mutually exclusive and implementing software solutions can be seen as a chief enabler for improving operations and analysis. In fact, more inefficiencies may be introduced without automating the processes.
The advancers are using best practice processes as well as modern, adaptable MES to support those practices. Exactly why they are investing in plant operations can be summarized as follows:
Manufacturing is the key to value creation, and it is often hampered by paperwork that can be automated.
As the product line grows along with volumes, complexity in the plant skyrockets and people cannot expect to manage it manually.
Minutes can make a difference in whether a manufacturing process creates profit or hurts it, so automating the process can pay off in obvious ways.
Plant information can also help avoid the risk of creating off-spec or poorly documented product that can lead to regulatory noncompliance, fines, or even recalls.
Rising demand and rising innovation are squeezing production operations, so they need to be able to improve as rapidly as possible.
There could be dozens of specific reasons a company would invest in improving plant operations. Many of those can be addressed with sound process design and MES to support those processes and the employees who execute the manufacturing processes. Think about it–can your plant operations remain competitive as the Advancers invest?
About the Author(s)
You May Also Like