Avenu Medical said that Ellipsys is a disruptive technology and provides a connection to veins and arteries in patients who need hemodialysis.
June 27, 2018
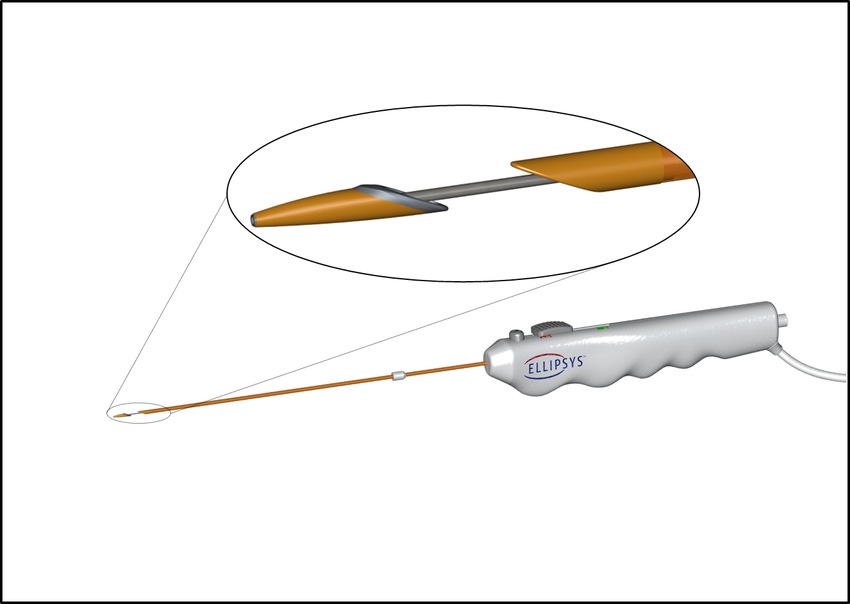
A new catheter-based technology could provide a non-surgical option for arteriovenous (AV) fistula creation. FDA recently gave de novo clearance to Avenu Medical’s Ellipsys Vascular Access System.
The San Juan Capistrano, CA-based company’s device challenges the surgical option to create an AV fistula, which has been the gold standard for about 50 years.
“This is what we call a disruptive technology for sure,” Ed Chang, Co-Founder, Director and VP Marketing for Avenu, told MD+DI. “We liken this to lap chole. Prior to lap chole it was always done surgically. That was the gold standard until laparscopic technology entered the system. We believe the same thing is going to happen with the Avenu technology with percutaneous AV Fistula creation.”
The device can make an AV fistula or a connection to veins and arteries in patients with chronic kidney disease who need hemodialysis. In Europe where the technology has been available since 2016, patients call the Ellipsys procedure the “scarless fistula.”
"What we’ve done at Avenu is come up with an AV fistula without surgery,” Chang said. “We go in with a single catheter venous access and we go in and under ultrasound guidance we create the fistula. We actually fuse the vein and artery together and we quickly are able to remove the catheter and the fistula is formed without doing a surgery… you walk out with a band aid.”
FDA reviewed data from a non-randomized, U.S. multi-center study of 103 patients to clear the technology. Avenu isn’t the only company to receive approval from FDA. TVA Medical also received a nod from the agency for the everlinQ endoAVF System.
“Dialysis is a necessary and life-saving procedure for thousands of individuals,” said Bram Zuckerman, M.D., director of the Division of Cardiovascular Devices in FDA’s Center for Devices and Radiological Health, in a release. “With today’s action, there will be additional, less-invasive vascular access options for patients who will require hemodialysis.”
About the Author(s)
You May Also Like




