July 13, 2015
The acquisition of CardiAQ Valve Technologies further boosts Edwards strategy to expand transcatheter valve replacement options.
Chris Newmarker
|
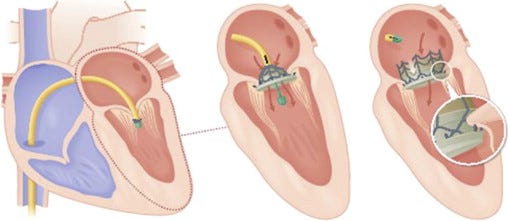
The CardiAQ device is theaded into the heart from below. |
Edwards Lifesciences will spend up to $400 million to acquire CardiAQ Valve Technologies and its transcatheter mitral valve replacement system.
The deal, announced Friday evening, should provide a boost for Edwards, which was already working on its own Fortis mitral replacement system. Both companies are based in Irvine, CA.
"We believe the experiences and technologies of Fortis and CardiAQ are complementary and that this combination will enable important advancements for patients," Michael A. Mussallem, Edwards' chairman and CEO, said in a news release.
Edwards plans to pay $350 million at closing, with another $50 million paid upon achievement of a European regulatory milestone. The deal is still subject to customary closing conditions, but Edwards expects it to close soon enough to be dilutive to 2015 earnings per share.
Edwards and Medtronic are the two market leaders in transcatheter aortic valve replacement. Even with Medtronic's entry into the U.S. market last year with its CoreValve, Edwards expects sales of its Sapien valve technology to grow by up to 25% this year, Mussallem said in an April earnings call with analysts, transcribed by Seeking Alpha.
Last month, Edwards announced FDA approval of its Sapien 3 transcatheter heart valve, the latest version of its best-selling Sapien valve that first won approval in 2011.
The Sapien 3 has a major design change in that it adds a skirt at the base of the valve to minimize leakage around the valve.
Meanwhile, Medtronic has won an expanded FDA indication for the CoreValve that allows it to be used in patients whose existing artificial heart valves are failing, but are considered too high risk for traditional open-heart surgery.
The CardiAQ acquisition could be another way for Edwards to potentially gain an edge on Medtronic in the field, by expanding it to replacement of other valves in the human heart. CardiAQ already has an FDA Investigational Device Exemption approval to conduct an early feasibility study of up to 20 patients, and plans to initiate a CE Mark study in Europe.
RBC Capital and Northland Capital Markets both upgraded Edwards shares to Outperform amid news of the acquisition, with Edwards stock closing at nearly $152 per share on Monday, up about 3%.
Interestingly enough, CardiAQ actually has a CoreValve link. Its CEO in recent years has been Rob Michiels, who was a president, chief operating officer and director at Irvine, CA-based CoreValve. (Medtronic acquired Irvine, CA-based CoreValve in 2009.) CardiAQ's leadership page on its website also lists Stan Komatsu, PhD, as acting vice president of valve operations. Komatsu was former vice president of valve operations at CoreValve, and was also a vice president of heart valve manufacturing at Edwards.
OrbiMed led CardiAQ's $37.3 million series B financing in 2012, and is CardiAQ's largest shareholder. Other venture investors include Broadview, Advent, and Versant.
Refresh your medical device industry knowledge at MEDevice San Diego, September 1-2, 2015. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
