This quick guide will help you determine if your app can slip through the regulatory cracks or if it must face FDA scrutiny.
August 6, 2014
Mobile medical applications are one of the hottest health trends going. More than half a billion smartphone users are expected to be using mobile medical apps next year, and that number will climb to more than 1.7 billion by 2018, according to a report from Research2Guidance.
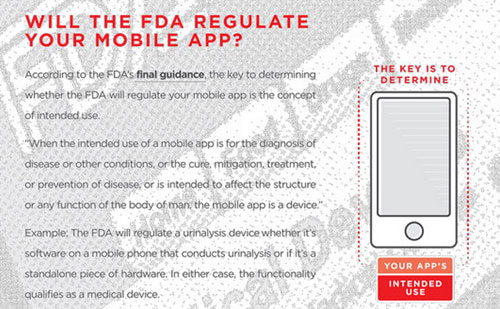
It's no surprise, then, that the medical device industry is becoming increasingly interested in developing these apps. But FDA, too, has taken notice. In September 2013, the agency issued its Mobile Medical Applications Guidance for Industry and Staff to explain how it would approach regulation of this emerging field.
MD+DI editorial advisory board member Bradley Merrill Thompson, a member of law firm Epstein Becker & Green and head of the firm's Connected Health Initiative, has been following FDA's progress on mobile apps regulation. Together with Worrell, a product design, development, and strategy firm that collaborates with medical device companies and entrepreneurs, he developed the following infographic guide to help developers quickly and easily determine if their mobile medical apps can slip through the regulatory cracks or if they must face FDA scrutiny.

—Jamie Hartford, managing editor, MD+DI
[email protected]
You May Also Like


