The San Diego,CA-based company said FDA has cleared the t:slim X2 insulin pump with Control-IQ technology, which integrates the DexCom G6 continuous glucose monitoring sensor.
December 17, 2019
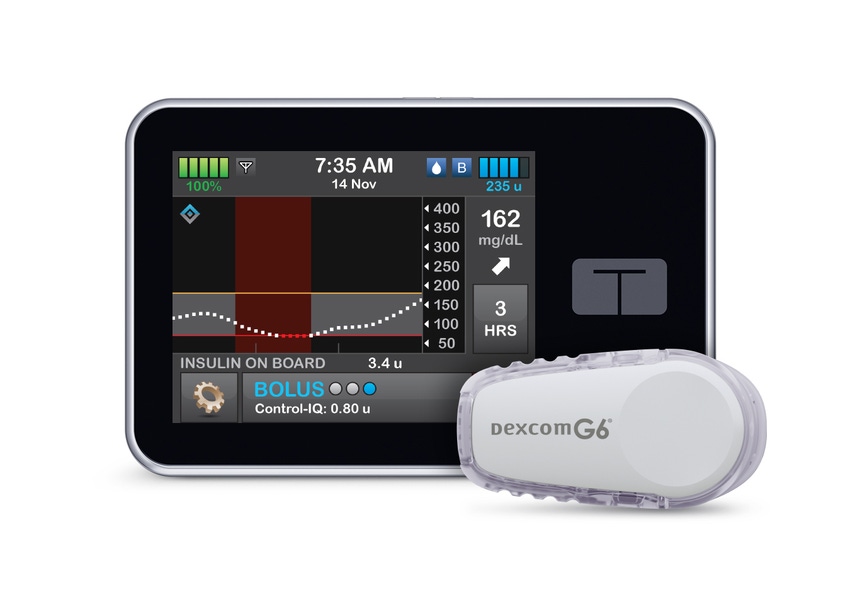
Tandem Diabetes has won a major nod from FDA that has the potential to advance its partnership with DexCom. The San Diego, CA-based company received clearance for the t:slim X2 insulin pump with Control-IQ technology, which integrates with Dexcom G6 continuous glucose monitoring (CGM) sensor.
The technology uses the DexCom G6 CGM sensor values to forecast glucose levels and adjust insulin delivery to ensure the prevention of erratic highs and lows. San Diego, CA-based DexCom’s CGM requires no fingersticks for calibration or diabetes treatment decisions.
Tandem first won FDA approval for the t:slim X2 insulin pump earlier this year. The approval was significant because it created a new device category – Alternate Controller Enabled Infusion Pumps.
The Control-IQ technology for the t:slim X2 insulin pump is an automated insulin dosing software in a new interoperable automated glycemic controller category that automatically adjusts insulin delivery to a person with diabetes by connecting to an alternate controller-enabled insulin pump (ACE pump) and integrated continuous glucose monitor (iCGM).
“Approval of the Control-IQ system with Dexcom G6 CGM brings together two incredible products to deliver a powerful automated insulin dosing solution for people with diabetes,” Kevin Sayer, president and CEO of Dexcom, said in a release. “Sensor accuracy is a critical component for automated insulin dosing, and we are excited that Dexcom G6 users can benefit from our collaborative effort to integrate this advanced hybrid closed loop system.”
This is the third category classified by FDA for the interoperability of devices as a complete automated insulin dosing (AID) system.
Tandem noted in-warranty t:slim X2 pump users in the U.S. will have the option to add the new feature free of charge via a remote software update. The update is expected to be available by the end of January 2020, and new pumps with Control-IQ technology will begin shipping to customers in the same timeframe.
“With this clearance, we will be launching the most advanced automated insulin dosing system commercially available in the world today,” John Sheridan, president and CEO of Tandem Diabetes Care said in a release. “This is a testament to our commitment to improving the lives of people with diabetes by offering simple-to-use products that deliver superior performance.”
Tandem’s most recent nod comes a few months after the company announced a partnership with Abbott Laboratories. The pact calls for the two companies to develop integrated diabetes solutions that combine Abbott's glucose-sensing technology with Tandem's insulin delivery systems to provide more options for people to manage their diabetes.
When news broke in October about the partnership, shares of Tandem shot up nearly 9.5%, however, DexCom shares slipped 4.5%. At the time DexCom investors appear to see the deal as a competitive threat to DexCom's G6 CGM device.
About the Author(s)
You May Also Like




