July 9, 2014
MicroCHIPS, a Lexington, MA-based startup backed by the Gates Foundation, is hoping to begin preclinical testing of its wireless remote-controlled contraceptive in 2015. The proposed device is projected to deliver a daily dose of 30 micrograms of levonorgestrel, an already-approved female contraceptive, for 16 years.
|
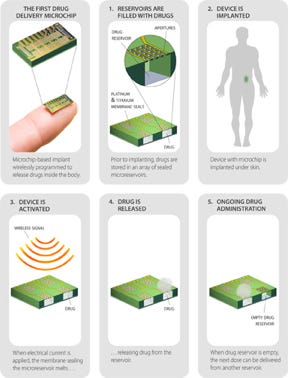
MicroCHIPS infographic explains their technology. (Courtesy MicroCHIPS) |
The company first demonstrated its individual microreservoir technology in a paper in the journal Science Translational Medicine, "First-in-Human Testing of a Wirelessly Controlled Drug Delivery Microchip." In that study, human parathyroid hormone fragment [hPTH(1-34)], the only approved anabolic osteoporosis treatment, was programmed to be delivered in escalating doses to osteoporotic postmenopausal women for 4 months. The controller was wirelessly programmed to release doses from the device once daily for up to 20 days.
In the abstract, the authors reported that "(d)evice dosing produced similar pharmacokinetics to multiple injections, and had lower coefficients of variation. Bone marker evaluation indicated that daily release from the device increased bone formation."
The MicroCHIPS technology for its remote-controlled contraception device hermetically seals discrete doses of the levonorgestrel in each microreservoir. The company says that precise long-term drug delivery can be achieved by using its individual microreservoirs to store and protect the drug. A microchip activates to release the drug, and telemetry both controls and communicates release of the drug. That release can be turned on and off with the wireless remote control.
MicroCHIPS says its technology is based on its proprietary reservoir arrays that are used to store and protect drugs within the body for long periods of time. These arrays are designed for compatibility with preprogrammed microprocessors, wireless telemetry, or sensor feedback loops to provide active control.
According to MIT Technology Review, MicroCHIPS invented a hermetic titanium and platinum seal for their reservoirs. Passing an electric current through the seal from an internal battery melts it temporarily, allowing a small dose of the hormone to diffuse out each day.
"The idea of using a thin membrane like an electric fuse was the most challenging and the most creative problem we had to solve," MicroCHIPS president Robert Farra said. The company says its goal is to achieve FDA approval by 2018.
Refresh your medical device industry knowledge at MEDevice San Diego, September 10-11, 2014. |
Stephen Levy is a contributor to Qmed and MPMN.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like