Medical device manufacturers can employ SaMD to develop smart care systems that are easy to deploy, configurable, and interoperable.

Over the years, patient care delivery has transitioned from simple sense measurement (touch, smell, sight) to more complex devices and thereafter to smart software systems. This shift aims to improve screening, diagnosis, treatment, and clinical management. The journey got catapulted roughly in 2003 when Zephyr came up with one of the first commercially available wearable, wireless, field-deployable monitoring systems. 1 Technology advancements enabled the gradual shift toward professional-grade monitoring systems for telehealth and remote patient monitoring markets. Finally, we are at the cusp of the new-age health ecosystem comprising portable point-of-care, remote anytime-anywhere-care, and next-gen patient-assistive devices. This advancement has naturally been accompanied by a shift of risk on patient safety from human errors to technical errors, which needs to be managed and mitigated efficiently.
Why Software as a Medical Device (SaMD)
Software as a medical device (SaMD) is expected to be the key driver for this next wave of medtech innovation worldwide. A majority of medical device organizations are creating infrastructures to support, implement, and embrace SaMD. A robust and future-proof SaMD strategy is predicted to be an important differentiator for medical device organizations. This can be realized by using technology levers in areas of digital health, IoMT (internet of medical things), and AI-ML (artificial intelligence and machine learning), further fueled by industry megatrends of continued focus on patient-centricity and improved health outcomes. It is therefore not a surprise that the SaMD market is estimated to grow at an annual growth rate of 22% till 2027. 2
Value Additions
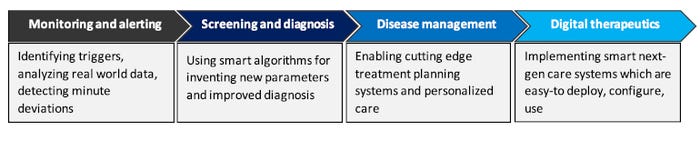
The key areas where SaMD has potential to generate high value for stakeholders (patients, healthcare providers and device manufacturers) across the value chain are:
Monitoring and alerting
Screening and diagnosis
Disease management
Digital therapeutics
These value additions might range from improved monitoring by identifying triggers, analyzing real-world data, and detecting minute deviations, to using smart algorithms for improved diagnosis, enabling cutting-edge treatment planning systems, and personalized care. Using SaMD, device manufacturers can make next-gen smart care systems that are easy to deploy, configurable, and interoperable.
Use Examples
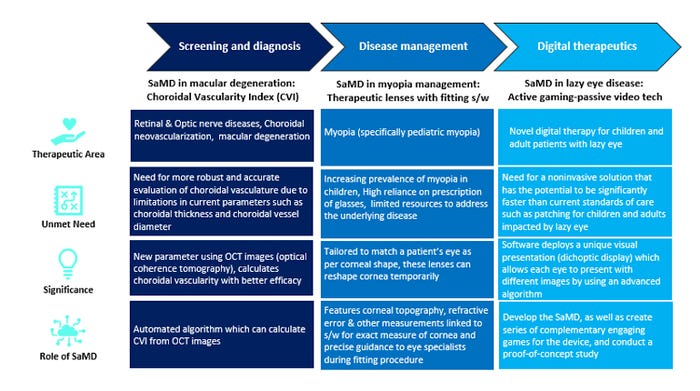
Diving deep into a therapeutic area and taking examples from ophthalmology domain, SaMD has use cases across the value chain, as described in the two tables and list below.
Under Screening and Diagnosis, SaMD has enabled a new diagnostic parameter for macular degeneration cases and is helping to track choroidal neovascularization for patients with retinal and optic nerve diseases, especially cases of severe or wet macular degeneration. The earlier diagnostic parameters have certain limitations. The SaMD technology helps overcome these gaps using optical coherence tomography to calculate choroidal vascularity.
Another example of SaMD usage in ophthalmology under Disease Management is its use in the field of myopia management. SaMD provides specialists with an efficient and intuitive software for fitting therapeutic lenses onto the patient’s eye, by providing accurate measurements of patient’s retina and enabling precise fitting procedure
Under Digital Therapeutics in ophthalmology, SaMD has enabled a smart treatment for lazy eye disease patients, especially kids. Lazy eye disease affects approximately 3 out of every 100 children worldwide. One of the current standards of care is patching, which is invasive in nature. Researchers have developed an advanced algorithm for a gamification software (SaMD) that uses dichoptic display technology, thus acting as a non-invasive disease management solution. 3,4
SaMD Advancements and Other Work-in-Progress Examples
There are numerous examples of SaMD in other areas as well that are currently under research and development. One impactful SaMD usage is related to stroke management. Stroke is one of the leading causes of mortality and morbidity globally. Diagnosis time and accuracy are of utmost importance to manage stroke patients and can drastically improve outcomes. Healthcare providers in emergency departments need tools and techniques that can enable them to identify the underlying cause (ischemic versus hemorrhagic stroke) quickly and accurately, since it decides the further line of treatment. Multiple research initiatives are ongoing in this domain leveraging smart algorithms, imaging solutions, and AI-ML to develop SaMD that can potentially revolutionize the response to stroke. 5,6
SaMD is also being applied in the pediatric ear care domain to diagnose ear infections and otitis, which are common but tough to diagnose among children. Children have been accurately and easily screened for acute otitis using a smartphone camera with an otoscope attachment and a platform-agnostic algorithm (SaMD). Researchers have also developed an application to detect presence of fluid inside ear using the smartphone’s microphone and speaker. 7,8
A recent SaMD technology is one of the world’s first non-invasive, prick-less saliva-based glucose test for diabetes management that measures glucose in saliva, using a glucose biosensor unit and a digital healthcare app. Similar work is in progress for a patch-based glucose sensing. 9
Conclusions
With the advent of SaMD, non-medical device manufacturers that are essentially software developers are also becoming part of an already complex, stringent, and fast-changing medical device regulatory ecosystem. This has direct and indirect implications on safety and risk of the software. Several international and regional regulatory bodies are collaborating to create a robust, standardized, and harmonized regulatory pathway for SaMD, since all stakeholders realize that the scope of SaMD is huge. 10 It has the potential to be leveraged across therapeutic areas and domains and is a promising proposition for device manufacturers, health providers, technology partners, and, most importantly, patients. It is important that medical device manufacturers acknowledge SaMD as an additional lever to expand the dimensions of their offerings and maximize their value potential in improving care delivery and health outcomes.
References
Medtronic, History and The Future, Zephyr Performance Systems, zephyranywhere.com.
"How to Correct a Lazy Eye," Healthline, https://www.healthline.com/health/how-to-fix-lazy-eye
"Novartis buys Amblyotech to develop digital therapy for lazy eye condition," NS Medical Devices, https://www.nsmedicaldevices.com/news/novartis-amblyotech-lazy-eye/
Zahoor, Arbaaz, "Computer Aided Diagnosis of Stroke from Brain CT Images," IJSRD - International Journal for Scientific Research & Development Vol. 3, Issue 05, 2015, ISSN (online): 2321-0613, www.ijsrd.com/articles/IJSRDV3I50666.pdf
"Deep Learning in Medical Diagnosis: How AI Saves Lives and Cuts Treatment Costs," AltexSoft, https://www.altexsoft.com/blog/deep-learning-medical-diagnosis/#early-stroke-diagnosis-from-head-ct-scans.
Mousseau S, Lapointe A, Gravel J. "Diagnosing acute otitis media using a smartphone otoscope; a randomized controlled trial," Am J Emerg Med. 2018 Oct;36(10):1796-1801. doi: 10.1016/j.ajem.2018.01.093. https://pubmed.ncbi.nlm.nih.gov/29544905/
McQuate, Sarah, "First smartphone app that can hear ear infections in children," UW News, https://www.washington.edu/news/2019/05/15/smartphone-app-can-hear-ear-infections/
GBS Inc., https://gbs.inc/saliva-glucose/
Hua, Andy, "Regulatory Challenges of Software as a Medical Device (SaMD), Supporting Innovation AND Safety," DIA Global Forum, https://globalforum.diaglobal.org/issue/december-2019/regulatory-challenges-of-software-as-a-medical-device-samd/
About the Author(s)
You May Also Like






