Swing Therapeutics has emerged from stealth mode with the achievement of several key business and regulatory milestones.
August 18, 2021
Swing Therapeutics, a new San Francisco, CA-based digital therapeutics company, is taking a "swing" at fibromyalgia with an exclusive license from the university of Manitoba, a breakthrough device designation from FDA, VC funding, and the launch of a real-world study.
Swing is one of a growing number of companies exploring the digital therapeutics market, which has gone absolutely gangbusters this year.
The company's real-world study is based on a clinically validated program that it licensed from the University of Manitoba. Swing launched with a $9 million seed investment from JAZZ Venture Partners, a firm that has made a name for itself as a lead investor in the digital therapeutics space, if a look at its company roster is any indication.
Swing’s program is designed to digitally deliver acceptance and commitment therapy, a form of cognitive behavioral therapy. Swing is leveraging existing clinically validated approaches to treatment as well as completing its own clinical trials specifically to show the safety and efficacy of its program. In addition to a completed pilot study and the recently-launched real-world study, Swing is planning a multicenter, randomized controlled trial to support FDA clearance for its solution.
Unlike conditions where there is an identifiable trigger such as arthritis or an injury, fibromyalgia often arises without a cause. In patients suffering from this condition, the brain is perceiving pain that doesn't have an identifiable trigger point.
"So it's really debilitating. Even beyond the pain, patients suffer from sleeplessness, reduced physical function, difficulty concentrating, and the mental health issues that could come along with it, like anxiety and depression," Mike Rosenbluth, PhD, founder and CEO of Swing Therapeutics, told MD+DI.
While there are some drugs that help some fibromyalgia patients, those come with significant side effects and are not curative, so managing the condition continues to be a challenge for patients, he said.
“Behavioral therapies have evidence supporting their effectiveness for pain management, but are not widely available or easily accessed by the average individual. Swing is tackling the challenge of making these therapies more available in a digital format. I look forward to following Swing as the company continues to build evidence to support its approach,” said David Williams, PhD, associate director of the Chronic Pain and Fatigue Research Center at the University of Michigan, and advisor to Swing Therapeutics.
Swing’s daily digital therapeutic is designed to include lessons and interactive exercises aimed at helping patients apply well-established principles to their unique circumstances and gradually develop the ability to manage their condition. The self-guided program is intended to foster understanding and acceptance of the participants’ own symptoms, while teaching powerful condition management skills. The program being studied also incorporates a tool to help users triage symptom flares—a common occurrence with fibromyalgia. The core program lasts 12 weeks, with a maintenance mode for extended use.
Rosenbluth said the program challenges patients to accept their thoughts and feelings rather than trying to suppress or ignore them. He said Swing's digital therapeutics solution is designed to teach patient self-care skills like mindfulness, and with that, hopefully, comes an improvement in their ability to function, and ideally, a reduction of their symptoms.
“We believe that our engaging digital patient-centered program will significantly increase access to proven approaches that previously very few people have been able to experience,” said Mike Rosenbluth, PhD, founder and CEO of Swing Therapeutics.
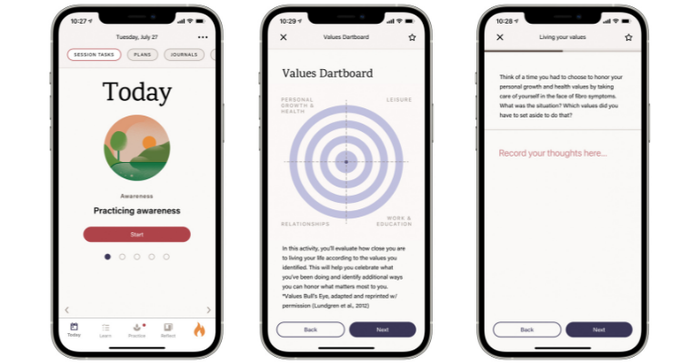
The images below show examples of ways the self-guided program encourages users to practice mindfullness and uses their own values to motivate behavior changes.
What are some driving factors behind the digital therapeutics boom?
One reason there has been so much interest in digital therapeutics, Rosenbluth said, is that there are so many conditions, like fibromyalgia, that are underserved with current solutions.
"There's just this real gap between what patients need and what's available," he said. "So I think a lot of the areas that you're seeing digital therapies start to emerge are some of those ones that typically have not been well served by drugs."
There's also a growing recognition of the need for access and sale, Rosenbluth said.
Another factor is the broader acceptance of digital health solutions overall, which has been one of the silver linings of the pandemic. COVID-19 has shown the healthcare system at large that it is possible to provide adequate, if not better, care than what was available through the traditional healthcare model. This is a trend that many people understood before the pandemic could and should happen, but there were so many hurdles preventing digital therapies from really taking off before 2020.
Down the road, Swing anticipates studying the use of its technology for other clinical applications such as autoimmune and chronic overlapping pain conditions.
The main difference between digital therapeutics and other sectors within digital health is that digital therapeutics are generally expected to be evidence-based, regulated technologies designed to trigger changes in patient behavior. Digital health products are not necessarily evidence-based or regulated.
"We as a company are really committed to evidence development," Rosenbluth said. "...One of the areas that we're really focused on is not only do patients benefit from this and physicians can prescribe it, but that payers will pay for it. I think the way to do that is with evidence."
Recent digital therapeutics financings and business developments
With so much activity happening as of late in the digital therapeutics market, here is a summary of some recent developments in the space:
San Francisco, CA-based Woebot Health closed a $90 million series B funding round, bringing total investment in the company to $114 million. Woebot says it has created relational technologies that underpin a new generation of digital therapeutics and tools for mental health. The company’s relational agent, Woebot, is designed to quickly form a bond with users and deliver human-like therapeutic encounters that are psychologically related, responsive to a person’s dynamic state of health, and targeted using multidisciplinary tools.
San Francisco, CA-based Mahana Therapeutics, a company developing a prescription digital therapeutic for irritable bowel syndrome that employs the use of cognitive-behavioral therapy, recently raised $61 million in a series B round. The company has raised about $81 million since its inception two years ago.
MedRhythms secured $25 million in series B funding to advance its digital therapeutics platform. The Portland, ME-based company is developing prescription digital therapeutics solution that use sensors, software, and music to measure and improve walking for patients with a neurologic injury or disease.
Berlin, Germany-based Dopavision closed a €12 million series A round in July to fund the clinical development of MyopiaX, its lead digital therapeutic in childhood myopia, with the goal to demonstrate its safety and efficacy in clinical studies.
Dario Health has also made a name for itself in the digital therapeutics field. New York, NY-based Dario provides adaptive, personalized experiences that drive behavior change through evidence-based interventions, intuitive, clinically proven digital tools, high-quality software, and coaching to help individuals improve health and sustain meaningful outcomes. The company secured $28.6 million in a private placement last year.
Another trend MD+DI has been tracking is the increasing number of medtech companies going public via a special-purpose acquisition company (SPAC), and there have certainly been a fair number of SPAC mergers involving digital therapeutics companies. The most recent example of this is Pear Therapeutics. Pear was founded in 2013, and has three FDA-cleared prescription digital therapeutic (PDT) products — reSET and reSET-O, and Somryst.
About the Author(s)
You May Also Like




