Nanowear has developed SimpleSense, a multi-parameter remote diagnostic undergarment, and machine learning digital platform, which simultaneously and synchronously monitors and assesses the heart, lungs, and upper vascular system.
November 30, 2020
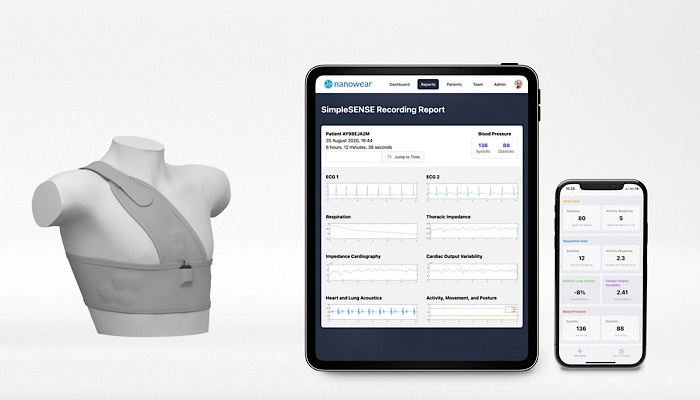
There’s little to no doubt that COVID-19 has given a shot in the arm to the digital health and telehealth market. Nanowear, a company that has developed a connected-care and remote diagnostic platform and plays in several digital health markets has seen the increased attention in the space.
Last month, the New York-based company won a nod from FDA for SimpleSense, a cloth-based diagnostic platform. The technology is a multi-parameter remote diagnostic undergarment and machine learning digital platform, which simultaneously, and synchronously monitors and assesses the heart, lungs, and upper vascular system.
Venk Varadan, co-founder and CEO of Nanowear said FDA clearance of SimpleSense comes at a time when demand is high for digital health and remote monitoring solutions.
“Pre-COVID our tech was super cool, and everyone could say, hey this is going to transform the game one day with all the different applications the platform can do,” Varadan told MD+DI. “But when COVID hit people were calling us up and saying, ‘hey we need your [technology] yesterday.’”
He added, “I think it accelerated everyone’s need to look at digital and remote solutions around telemedicine. COVID gave the whole world that shock that it needed to evaluate innovation seriously and not look as this is cool, as opposed to I need to implement this in practice.”
The company said the SimpleSENSE platform is Gender-neutral and size adjustable and has the potential to replace the digital stethoscope, multi-channel Holter monitor, Capnogram respiration machine, and blood pressure cuff by providing a diagnostic quality monitoring system that remotely captures more than 100 million data points per patient per day across cardiac, pulmonary, and circulatory biomarkers.
“We have billions of these nanosensors per centimeter of surface area,” Varadan said. “It can capture really low-frequency biomarkers from basic skin contact – without the need for gel, wires or anything like that.”
He added, “what it really results in is the ability to pick up clinical-grade biomarkers from the skin over the same period – synchronously and continuously.”
In addition to the near-term commercialization of SimpleSENSE with select channel partners, Nanowear plans to continue its SimpleSENSE clinical trials in diagnosing worsening Heart Failure and COVID-19.
The company is in a unique position because its solution crosses over into multiple markets.
“I get this question all of the time. Are you a device company; are you a data company; or are you a telehealth company,” Varadan said. “That’s an antiquated way of looking at this space. You can’t bucket a technology like ours into one of three or four of those categories.”
He added Nanowear differs from other companies because it monitors more than one biometric.
However, companies focused on measuring single sets of biometrics that have been making a lot of noise recently. Companies like AliveCor and IRhythm are examples of these firms.
A few weeks ago, Mountain View, CA-based AliveCor raised about $65 million in a series E round. AliveCor also announced former Cleveland Clinic CEO Toby Cosgrove, MD was joining its board. The company sells the FDA-cleared KardiaMobile device, which is designed to provide instant detection of atrial fibrillation, bradycardia, tachycardia, and normal heart rhythm in an ECG.
iRhythm presented strong 3-year data from mSToPS, a study that evaluated the effectiveness of Zio, the company’s ambulatory monitoring patch, to detect silent, or previously undiagnosed, atrial fibrillation in moderate-risk individuals.
About the Author(s)
You May Also Like


