Digital health solutions working in FDA-regulated, clinical environments will save billions for the U.S. healthcare system in the future.
June 8, 2015
As digital solutions - both devices and software - move from the consumer field to the clinical domain, it's poised to benefit both patients and the overall healthcare industry.
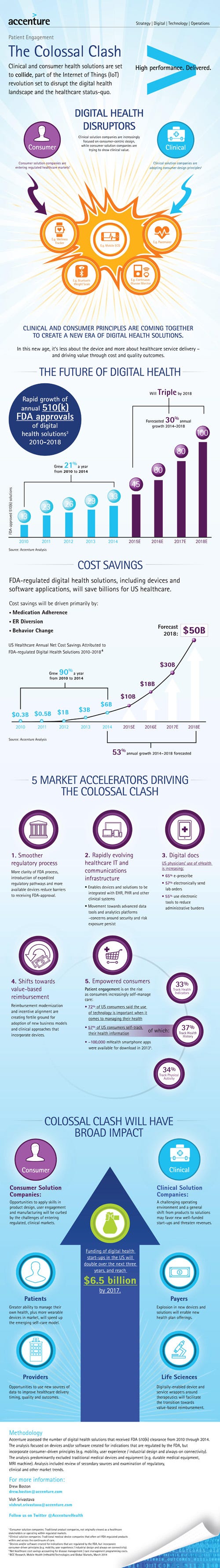
Research from Accenture estimates that the number of digital solutions - both devices and solutions - cleared by the FDA since 2014 will drive more than $100 billions in savings for the U.S. healthcare system.
The savings were driven by fewer visits to the emergency room, better adherence to medication and behavior change, according to Accenture. In 2014, the healthcare system saved $6 billion as a result of these digital solutions and each year after that is expected to see greater savings - $10 billion this year, $18 billion in 2016, $30 billion in 2017 and $50 billion in 2018.
The increase in savings is commensurate with a rise in the number of FDA-cleared digital solutions over the years. FDA cleared 33 digital solutions in 2014, up from just 13 in 2010, according to Accenture. The consulting firm projects that 45 separate digital offerings will reach that milestone this year and projects the number of FDA-cleared digital solutions to jump to 100 in 2018.
”The proliferation of internet-connected solutions and evolving regulatory guidelines are blurring the lines between clinical and consumer health solutions,” saidRick Ratliff, managing director of digital health solutions, Accenture, in a news release. “As consumer health platforms support more ‘medical’ devices, rather than just today’s wellness trackers, they’ll create a viable self-care model in a segment that today is occupied by chronic-disease monitoring companies.”
A new design paradigm is also being developed as consumer companies leverage their expertise with users and create devices for the regulated, medical world in an attempt to disrupt the status quo. At the same time, traditional healthcare and medtech companies with decades worth of experience in developing regulated devices adopt a consumer-centric design approach to develop medical devices that are easier to use.
Accenture is describing this phenomenon as the Colossal Clash. The net result is hopefully better outcomes for patients.
“A digital disruption is playing out in healthcare, as witnessed by the emergence of new business models and technology that will change the nature of patient interactions, alter consumer expectations and ultimately improve health outcomes,” Ratliff noted.
Here is the infographic that spells out the disruption:
Arundhati Parmar is senior editor at MD+DI. Reach her at [email protected] and on Twitter @aparmarbb
Stay abreast of industry trends at MD&M East Conference in New York, June 9-11 at the Jacob J. Javitz Convention Center. |
You May Also Like


