The company has agreed to acquire EOFlow, a South Korean company that makes a wearable insulin patch.
May 25, 2023
Now that Medtronic's diabetes business has resolved its warning letter and scored FDA approval for the MiniMed 780G system with the Guardian 4 sensor, the company is looking to M&A to further boost its diabetes pipeline.
Medtronic has agreed to acquire EOFlow, a South Korean company that makes a wearable insulin patch, for about $738 million at current exchange rates.
Seongnam, South Korea-based EOFlow makes the EOPatch, a tubeless, wearable, and fully disposable insulin delivery device. The company said the addition of EOFlow, combined with Medtronic's meal detection technology algorithm and continuous glucose monitor, is expected to expand the company's ability to address the needs of more individuals with diabetes. The deal is expected to close in the second half of the year.
"Our goal is to simplify diabetes management and deliver the well-established benefits of automated insulin delivery to our customers in the ways they want and need," said Que Dallara, EVP and president of Medtronic's diabetes business. "We're excited to introduce a differentiated wearable patch option to provide more patient choice and drive further innovation for those who want to use technology to make living with diabetes easier. We look forward to expanding our offerings to participate in the patch pump market and enabling those customers access to our seamless ecosystem of support."
The EOPatch device, which features a microfluidic technology designed to deliver insulin with high accuracy and reliability while minimizing the risk of insulin occlusion, is authorized for marketing in Europe, South Korea, Indonesia, and the United Arab Emirates with a compatible smartphone application that allows users to monitor and control the patch directly from their phone.
"Look, we have not blinked when it comes to diabetes, and we're shifting to offense as we continue to invest heavily in assembling our ecosystem of durable pumps, smart pens, patch pumps, sensors, algorithms, and customer service with multiple programs under development," CEO Geoff Martha said during Medtronic's fiscal fourth-quarter earnings call Thursday. "Having this ecosystem is really important because we believe the market will move from CGM first to automated insulin delivery, and we are well positioned for that trend."
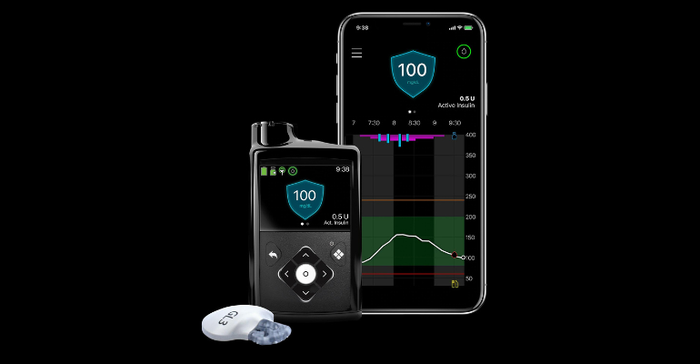
Medtronic said it will work to integrate the EOPatch device with its next-generation continuous glucose monitoring (CGM) sensor and meal detection technology algorithm currently offered in its MiniMed 780G system (pictured below) after the acquisition closes.
"We're thrilled to accelerate our next phase of growth with a partner like Medtronic who shares our goal of simplifying diabetes management to make life easier for the customers we serve," said Jesse J. Kim, CEO and founder of EOFlow. "Together, we'll work to advance innovation in wearable insulin patch technology to expand our reach to more individuals around the world living with diabetes. With a global footprint in over 100 countries, ability to scale up manufacturing quickly, and advanced software and sensor capabilities, Medtronic is the ideal strategic partner for EOFlow."
Financial details of the acquisition
Pursuant to share purchase agreements (SPA) with Jesse J. Kim (CEO) and Luis Malave (president), Medtronic will acquire all their shares in EOFlow at a price per share of KRW 30,000. Pursuant to a share subscription agreement (SSA) with EOFlow, Medtronic will acquire new shares at a price per share of KRW 24,359 to fund EOFlow's ongoing operational and research and development requirements. Medtronic will also undertake a public tender offer to acquire up to all outstanding public shares at a price per share of KRW 30,000. The public tender offer will be subject to a minimum condition such that after the closings under the SPA, SSA, and the public tender offer, which will occur on the same day, Medtronic will hold at least a majority of the shares outstanding on a fully diluted basis. With these transactions, Medtronic intends to acquire all outstanding shares in EOFlow and delist the company.
To the extent that all the public shares participate in the tender offer, the total consideration for the acquisition of the shares in EOFlow would be KRW 971 billion, or $738 million, at current exchange rates.
Impact on the competitive diabetes landscape
While the news itself didn't come as a big surprise (BTIG's Marie Thibault noted in a report today that Korean media outlets reported in February that Medtronic was interested in EOFlow), the impact the news had on shares of Insulet was a bit puzzling.
"We are somewhat perplexed to see Insulet shares down nearly 8% in intraday trading, since we thought this was a known risk and expected competitive noise. We think this is an overreaction," Thibault wrote. "We have followed EOFlow's progress for more than five years and expected it to enter the U.S. market with a domestic distributor over the medium-term."
The analyst notes, however, that EOFlow submitted a 510(k) to FDA for the EOPatch late last year. She also points out that Insulet filed a patent suit against the company's German distribution partner, which led to a preliminary injunction and suspension of sales in March.
"We think this may be a boon for [Medtronic's] diabetes pipeline, giving them an option away from tubed pumps, but do not think it is an immediate threat to [Insulet's] monopoly in patch pumps," Thibault wrote.
She added that there are still unknowns about how Medtronic's next-generation CGM sensor will perform against market-leading CGMs from Abbott and Dexcom.
"We also think that many patients will continue to prefer the open approach of mixing and matching CGMs with pumps, and that this may be a barrier to competitive switching from [Insulet] to an eventual [Medtronic] offering. Also, it will take some time to develop this closed-loop patch pump system and gain FDA clearance," Thibault wrote. "We continue to think [Insulet's] market development efforts, new areas for expansion, and large competitive lead will serve it well."
About the Author(s)
You May Also Like




