There are inconsistencies in the definitions of Normal Use and Abnormal Use. Here’s what should be corrected.
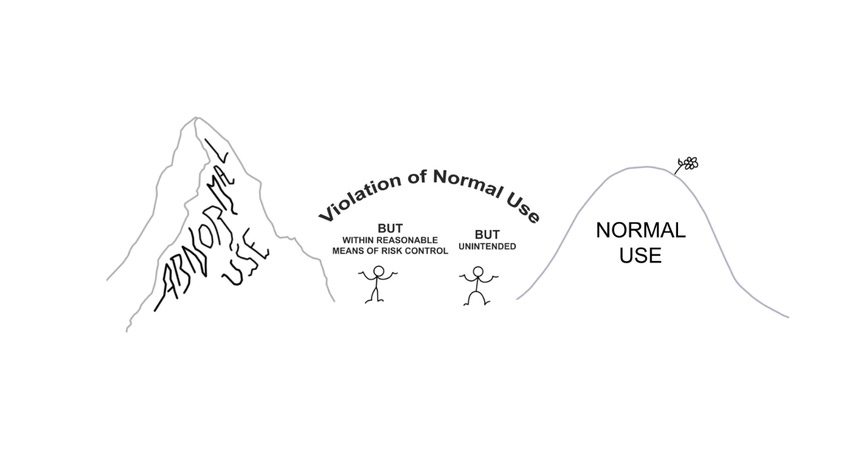
FDA and international human factors engineering guidelines agree that manufacturers can disregard Abnormal Use when it comes to medical product usability testing. However, the specific meaning of Normal Use and Abnormal Use differ between U.S. and international guidelines. This leaves medical product manufacturers with an unclear understanding of what is within scope versus out of scope of the usability engineering process.
As FDA’s 2016 guidance, “Applying Human Factors and Usability Engineering to Medical Devices,” describes it, Abnormal Use occurs when a user abuses or sabotages a device. The international usability engineering standard, IEC 62366, which was harmonized by the EU in 2008, accepts a much broader definition for Abnormal Use. It includes any intentional act or intentional omission of an act contrary to Normal Use.
The problem with IEC 62366 is that it forges a chasm between Normal Use and Abnormal Use in which the following two scenarios fall:
Violation of Normal Use due to unintentional actions.
Violation of Normal Use within reasonable means or risk control.
These two scenarios do not fit under the category of Normal Use or Abnormal Use (see Figure 1 above.)
This article offers a proposed solution for redefining medical device use in IEC 62366-1 to fully capture all instances of use and to better align with the FDA 2016 guidance.
Background on Medical Device Use and Usability
Though medical devices can provide life-saving treatment, they must be designed responsibly to ensure that they are safe to use. A study found that more than 250,000 deaths per year are caused by medical error, making it the third leading cause of death in the U.S.1
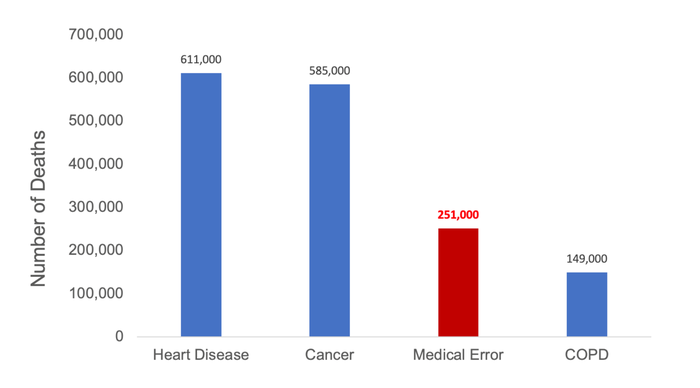
Figure 2: Top Causes of Death in the United States (2013)1
How does a manufacturer determine whether its medical device can be used safely? In the medical device regulatory world, IEC 62366-1 is the international standard for usability engineering of medical devices, and it includes usability requirements and considerations.2 In the U.S., FDA enforces a guidance they published in 2016.3
Manufacturers must demonstrate to these agencies that the device can be used safely and effectively in order for the device to be approved and subsequently distributed. Importantly, the usability of the device must be evaluated to ensure that:
Risk documentation adequately captures use errors.
Any use errors are subsequently evaluated and mitigated prior to market release.
FDA and IEC 62366-1 define medical device use differently, leading to manufacturer confusion. While IEC 62366-1 describes medical device use as either Normal Use or Abnormal Use, FDA defines only Abnormal Use and considers all other types of use as Correct Use or Use Error. To appropriately and safely develop medical devices, both guidelines should collectively present one complete and consistent description of use.
The Problem with IEC 62366-1 “Use” Definitions
IEC 62366-1:2015 implies that a medical device can be used only “normally” or “abnormally,” both through its definitions of these terms and by Figure 1 of this guidance. The current portrayal of medical device use by IEC 62366-1 is depicted by the following figure.4
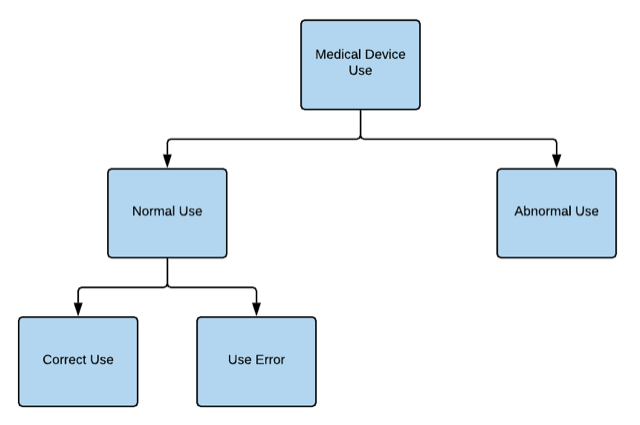
Figure 3: Medical Device Use as Portrayed by 62366-1:20154
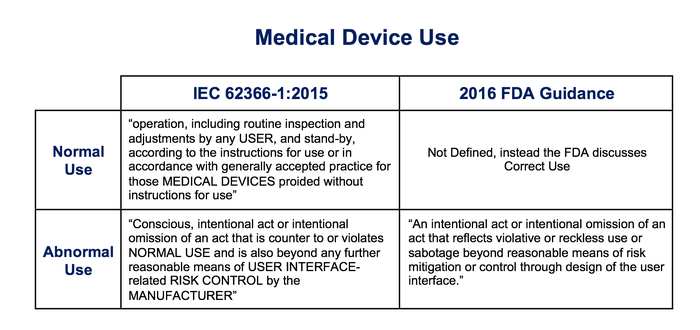
Figure 4: Definitions of Normal and Abnormal Use
The FDA guidance and IEC 62366-1 agree that Abnormal Use includes intentional actions that are beyond reasonable means of risk control by the manufacturer. However, the IEC 62366-1 definition of Abnormal Use is much broader than the FDA definition, covering all intentional violations of Normal Use. FDA only considers intentional abusive actions.
Additionally, IEC 62366-1 definitions of Normal Use and Abnormal Use do not cover all situations of medical device use, leaving a void for manufacturers to interpret.
According to IEC 62366-1, situations that are not Abnormal Use include:
Unintentional actions.
Intentional violation but within reasonable means of risk control.
Normal Use as defined by IEC 62366-1 is commensurate medical device operation “in accordance with generally accepted practice.” The term “normal” further implies a typical, average, or standard nature to use of the device.
Situations that are not “normal” or in accordance with generally accepted practice include:
Unexpected use by an unintended user.
Unexpected off-label use.
A qualified individual does something unexpected that is contrary to standard practice.
Gaps between the IEC 62366-1 definition of Normal Use and Abnormal Use as well as discrepancies between FDA and IEC 62366-1 can lead to confusion among manufacturers, causing them to disregard important use scenarios which lead to harm. Below we have identified two (2) undefined types of device use in IEC 62366-1.
Scenarios Not Covered by IEC 62366-1
1. A user unintentionally violates Normal Use
A user who unintentionally violates Normal Use would neither fit into Abnormal Use nor Normal Use. Situations that fall into this scenario include the following:
a. Unexpected, accidental use of a product by an unintended user or patient.
Example:
An over-the-counter (OTC) asthma rescue inhaler is intended for temporary relief of a mild/moderate asthma flare. However, a user experiencing a severe asthma attack unintentionally selects and uses this OTC product because the packaging/labeling is not well designed to indicate it is inappropriate for this use. The person uses the inhaler correctly, but it is not intended for use during a severe asthma attack, and thus it does not fall into the category of Normal Use. This is also not Abnormal Use, since the user has unintentionally chosen to use the inhaler.
b. Unintentional use of a product for an unexpected off-label purpose.
Example:
A trauma surgeon uses a bowel grasper intended for laparoscopic abdominal surgery during coronary surgery. The trauma surgeon is an intended user for this device; however, he/she is unintentionally using it for an off-label procedure leading to tissue damage. Since the procedure is off-label, this cannot be considered Normal Use, and since the doctor is using it unintentionally, it cannot be considered Abnormal Use.
c. A qualified individual unintentionally does something unexpected that is contrary to standard practice.
Example:
Cardiac ablation is a commonly used technique to treat atrial fibrillation (irregular heartbeat caused by problems in the electrical signaling of the cardiac conduction system). In this example, a cardiologist is feeling fatigued owing to working 12 hours straight. While setting the device intensity for an atrial fibrillation surgery, the cardiologist unintentionally increases device intensity above an acceptable level, leading to permanent damage to a patient’s heart. This situation cannot be considered Normal Use as the cardiologist should know not to use such a high energy; it is contrary to standard practice. It is also not Abnormal Use as this was an unintentional action.
2. The user intentionally violates Normal Use, but this action is within reasonable means of risk control.
Intentional use of a device that is contrary to Normal Use but reasonably foreseeable and within reasonable means of risk control by the manufacturer is not covered by IEC 62366-1:2015. Abnormal Use does not include Use Errors that are within reasonable means of risk control. Normal Use does not include medical device use contrary to the IFU or to generally accepted practice for use. Situations that fall into this scenario include the following:
a. Unexpected use of a product by an unintended user or patient that is within reasonable means of risk control.
Example:
A family member of the patient changes the settings on a mechanical ventilator even though she knew that only a technologist should change the settings. The family member is an unintended user for this product and consciously violated Normal Use by changing the settings. However, adding user logins could easily mitigate this concern, and thus cannot be considered Abnormal Use.
b. Use of a product for an unexpected off-label purpose that is within reasonable means of risk control.
Example:
A type 1 diabetic patient is able to hack and reprogram an insulin pump and glucose monitor so that he/she can automatically regulate insulin infusion throughout the day. Programming errors by the patient can cause an insulin overdose or underdose, which both could lead to death. This is an unexpected off-label use of both devices and thus cannot be considered Normal Use. Improving encryption technology would make these systems harder to hack and is within reasonable means of risk control and thus cannot be considered Abnormal Use.
c. A qualified individual does something unexpected that is contrary to standard practice but is within reasonable means of risk control.
Example:
An advanced life support (ALS) certified emergency medical technician (EMT) riding in an ambulance arrives at the scene of an emergency and uses a defibrillator they have not used before nor received training for, though they are trained and certified to use other defibrillators. The EMT consciously violated Normal Use by not being trained. Requiring a training log and signature prior to field work with any new defibrillator could easily mitigate this concern, and thus this situation cannot be considered Abnormal Use.
Discussion
Neither scenario can be placed into a category as described in IEC 62366-1 (see Figure 3). The disconnection between Abnormal Use and Normal Use creates a new type of medical device use that needs to be addressed.
Below we have illustrated the different scenarios for use of a medical device using an example, including those not currently covered by IEC 62366-1. The focus for this example is the task of shaking a pen injector for 10 seconds prior to injection in order to activate the medication.
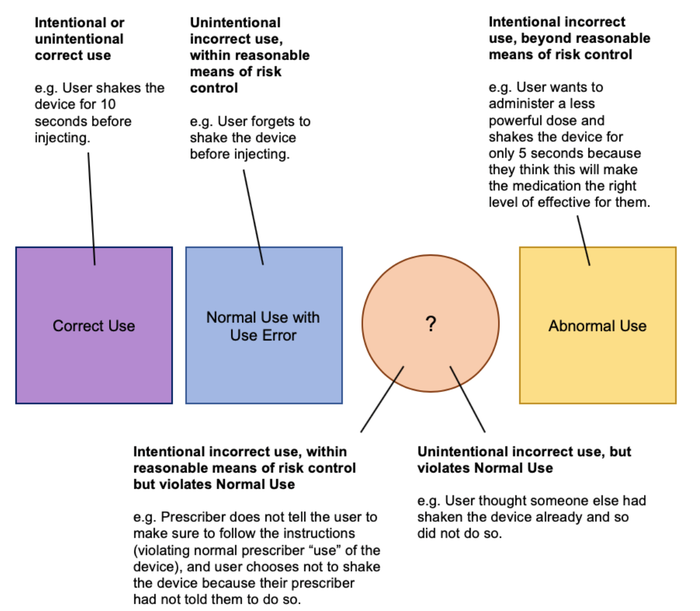
Figure 5: Medical Device Use Scenarios
This article demonstrates that IEC 62366-1 incorrectly omits use that is not Normal Use and does not fit into the definition of Abnormal Use. We propose to remove the term “Normal Use” from IEC 62366-1, to redefine Abnormal Use as abuse and further requiring removal of the “Relationships of the Types of Use” figure from IEC 62366-1. This would clarify that actions that are abuse are not considered “Normal” and that abuse is the only type of medical device use that is always out of the scope of risk control by the manufacturer.
This option would align with FDA’s 2016 guidance, which defines the safety and effectiveness of a device without defining Normal Use. It would make clear to manufacturers that use-related issues other than Use Errors and Abnormal Use should be examined.
At minimum we suggest updating the figure “Relationships of the Types of Use,” as this figure does not include the entire scope of medical device use. For example, we propose an updated hierarchy in Figure 6 below. Additionally, in the Scope section of the IEC 62366-1 standard, language would need to be added to clarify what should be done to address use cases that are neither Normal Use nor Abnormal Use.
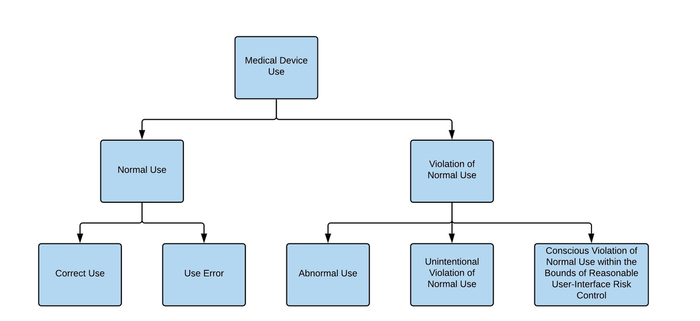
Figure 6: Proposed Update to the IEC 62366-1 Figure"Relationships of the Types of Use"
Conclusion
IEC 62366-1 leaves a chasm between Normal Use and Abnormal Use, so manufacturers might not consider some user actions to be “Use Errors” if these actions do not fit the IEC 62366-1 definition of use. In other words, Use Errors may go undetected, unaddressed, or unmitigated. To protect users and manufacturers, this gap must be closed: IEC 62366-1 should be updated to address user actions that do not fit the current definitions of Normal Use nor Abnormal Use. To mend this gap in IEC 62366-1, we suggest to:
Re-define Abnormal Use in terms of abuse.
Remove Normal Use.
Remove the “Relationships of the Types of Use” figure from the standard.
Thank you to Xin Feng, Human Factors Reviewer at the FDA Center for Devices and Radiological Health, for initiating this discussion during the April 2019 IEC Committee 62A Joint Working Group 4 meeting in Geneva, Switzerland, and for laying the groundwork for this article.
References
Makary, Martin. 2016. “Medical Error—the Third Leading Cause of Death in the US.” BMJ353.
IEC 62366-1:2015, “Medical devices – Part 1: Application of usability engineering to medical devices.”
FDA 2016, “Applying Human Factors and Usability Engineering to Medical Devices - Guidance for Industry and Food and Drug Administration Staff.”
IEC 62366-1:2015, “Medical devices – Part 1: Application of usability engineering to medical devices”
About the Author(s)
You May Also Like


