Nearly two decades ago, a bike ride inspired the design of what medical device?
April 25, 2023

When it comes to innovation, you just never know what will make your lightbulb go off. For Dan Jacobs, MD, that lightbulb moment occurred during a bike ride.
The Silicon Valley-based plastic surgeon was thinking about his breast reconstruction patients, and the painful process of needle injections with saline expanders, and he knew there must be a better way. As he stopped to fix a flat tire with a canister of compressed gas, Jacobs wondered if a similar device might be used inside of a tissue expander to make the tissue expansion process easier and more comfortable for breast reconstruction patients. He took the idea to his Silicon Valley contacts and AirXpanders was born in 2005 to develop the AeroForm.
The device represented the first major change in breast tissue expansion in almost 40 years.

About 11 years after that eureka moment, FDA granted de novo marketing authorization for AeroForm in 2016. By that point, the device had already received a CE mark in Europe in 2012 and Australian TGA certification in 2013.
Following a mastectomy, the AeroForm tissue expander is placed underneath the chest muscle to enable the chest wall to be stretched to make room for a permanent breast implant. The device contains a reservoir of compressed carbon dioxide. Using a hand-held, wireless dose controller, the patient can release 10cc of CO2, up to three times per day, to gradually inflate the expander. This can be done from home or work, and only takes a few seconds at a time. The system eliminates the need for weekly doctor visits and needle-based saline injections.
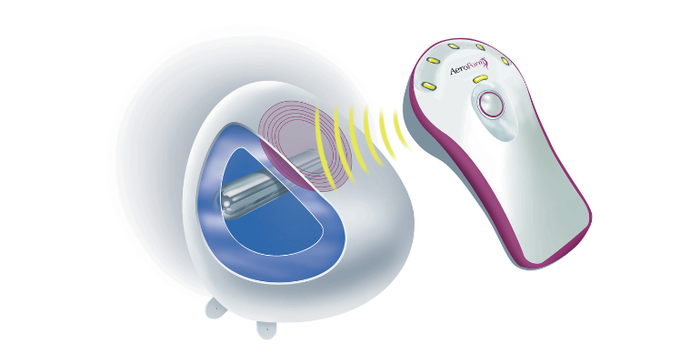
The air went out of AirXpanders
AirXpanders won Best in Show at the 2018 Medical Design Excellence Awards, but behind the scenes, the company was quickly losing air. AirXpanders laid off nearly all its staff and filed for bankruptcy in 2019. The news also followed FDA clearance of a smooth-shell version of AeroForm.
According to a May 12, 2021 order from the Securities and Exchange Commission, AirXpanders has not filed any periodic reports since 2019.
About the Author(s)
You May Also Like




