Medical device manufacturers could learn a lot from automakers about how to design products that are innovative and cost-effective.
March 4, 2013
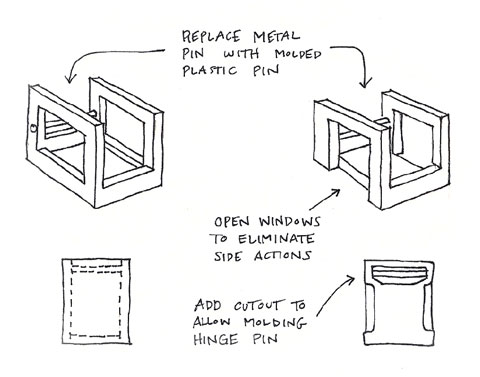
The medical device and automotive industries have a lot in common. They have similar performance and quality criteria, as they are both federally-regulated industries. Failure of medical devices or automotive components could ultimately cost a human life, so designing safe, effective components is vital. The two industries are also competitive, and innovation is one of the major keys to success in both.
Designing this part for manufacturability offered four advantages: two parts were reduced to one, part and tooling costs were reduced, a plastic pin reduced noise, and overall quality was improved. |
In the early 1990s, the automotive industry changed dramatically. The presence of global competition and the Big Three automakers’ lack of cost control created a situation that could have been damaging to the industry. The days of creating products without considering costs rapidly came to an end. For North American automotive companies to compete globally and be successful, they needed to start producing innovative and stylish cars at a lower cost.
Today, automotive innovation is at an all-time high, thanks to three factors:
Federal demand for vehicle safety, including crash avoidance (i.e., lane departure warning, forward crash warnings, fatigue warning, and vehicle-to-vehicle and vehicle-to-infrastructure communications) and safety restraint (i.e., head restraint, front air bags, side curtains, roll-over air bags, and retractor and safety belt pretensioners).
Federally-driven fuel efficiency standards to help reduce U.S. dependence on foreign oil, provide savings at the pump, and protect human health and the environment.
A growing consumer demand for in-vehicle technology that uses real-time information and smartphones (referred to as infotainment).
However, advances are ultimately adopted based on innovation, safety, reliability, and cost.
With all the recent changes in the healthcare industry resulting from increased competition and the Affordable Care Act, it is imperative that the medical device industry adopt many of the same practices the automotive industry uses to drive cost out of its products while introducing new, innovative solutions.
The automotive industry often works with experienced contract manufactuers and focuses on manufacturability. Effective, early collaboration is critical because a majority of any product’s total lifecycle costs are defined during the early phases of product development. The process should begin the first day of concept development. Prototypes and 510(k)s are often complete by the time manufacturing gets involved, leaving companies little room to implement quality improvements or cost-saving strategies. In both industries, it is usually too costly and time consuming to revalidate a component or material change after initial 510(k) approval. Working with these manufacturers reduces costs without sacrificing innovation.
Partner with Experts
Having a supplier that understands your product development process and can help overcome challenges lends itself to faster innovation. Even if you are already implementing the latest computer-aided process and product life cycle managment in-house, a professional firm that understands design services and how to manufacture the device ensures the final product will achieve its quality, performance, and cost targets.
A contract firm can do this by providing additional inputs on the following:
Concept development. Reviewing part of the geometry design with a manufacturing partner allows the designer to leverage the experts for manufacturing concepts. Most qualified suppliers can offer best practices concepts based on prior lessons learned.
Design and development. Working with a qualified partner from concept through final production can ensure your material selection meets performance needs, be readily available, allow for a robust production process, and keep costs in check.
Verification and validation. Prototyping and testing create valuable lessons for the manufacturer beyond the functionality of the device. If treated as an opportunity to learn about the device and the process, you can significantly increase your chances of streamlining production, reducing the time it takes to get your product to market, and ultimately keeping your capital investment low.
Study Component Design from a Manufacturing Standpoint
Two costs that stand out are materials and processing. Together, they typically account for 70% of the cost of goods sold. Focusing on materials will have the maximum impact.
Materials. In this context, materials stands for anything from purchased components, raw materials, or packaging. Every material has usually been specified, tested, and approved by the time a device is being planned for production. By the time it is ready to be manufactured, the firm awarded the business has limited flexibility in material selection. Achieving the lowest cost possible for the new device is difficult because the supplier is unable to select material from a variety of vendors to ensure the most cost-effective process from a minimum buy, logistics, and packaging perspective. In the design phase, the material companies’ sales rep will want the material called out by the trade name associated with it versus a generic specification spelling out what it is.
However, using generic specification rather than a specific grade gives the factory manufacturing your device the flexibility to make and submit the device with the most cost-effective selection. It also gives the manufacturer and company responsible for the design flexibility in switching suppliers down the road to take advantage of future cost savings or cost avoidances from vendor increases.
Even if a generic material type is selected, it may be cost prohibitive. Try a variety of different materials during your material selection process to ensure you are taking advantage of cost without limiting your functionality.
Another common pitfall when designing a medical device is that the minimum orders, locations, and availability of materials are not reviewed. The firm manufacturing the device will often find later that it is unable to get the price of the device down due to excessive freight cost, breakup charges, and large inventory increases, which tie up cash flow. If your device is projected to only use 1,000 lb per year of a certain grade of resin, it is imperative that a material is not selected that requires a large purchase. Typically a supplier would like to see a situation where it can stock a month or less of material and have a local distribution option. Selecting a vendor on another continent that requires a two-year supply of material will create an uncompetitive situation.
Processing. Process is anything from core manufacturing, quality, or any supporting process such as material handling.
Initial designs and testing are often done to create a product that achieves its performance standards, yet no thought is given to manufacturability or continuous quality. A device should be looked at from the perspective of how materials will be handled, how it will flow through a manufacturing process, and how it can be designed to increase production throughput and reduce labor needed to manufacture the device. The design must also be reviewed to ensure the parts can yield perfect quality. Poka-yokes, or designs that yield ease of assembly and reliability, are critical. There are three key steps to designing for manufacturability as follows:
Develop and evaluate multiple design concepts with the use of engineering analysis, rapid prototyping techniques, and general design skills.
Test and evaluate models to identify best practices in design, manufacturing, and performance.
Perform component and subsystem design to build analysis that aids in the comprehension of the manufacturability of a device for cost, process efficiency, and quality.
Involvement of your purchasing personnel early in the process is also critical. They will be able to help in the integration of the production suppliers to streamline the process of reviewing the device’s design for material selection, process, efficiencies, and quality. Purchasing personnel’s use of a strategic supply base will give them knowledge of all the suppliers’ capabilities and aid in the selection of the most qualified companies from knowledgeable engineering support, state-of-the-art manufacturing, and world-class quality. Ultimately, this collaboration will improve cost, quality, and business performance.
Integration of suppliers in the design phase of the process makes the supplier an extension of the firm, emphasizing continuity of design and the long-term success of the project. Purchasing personnel’s ability to link the correct suppliers will permit increased coordination at a tactical level, enabling the designer to effectively streamline the design to bring an innovative, high-quality device to market while ensuring manufacturing costs are minimized and profits are maximized. The approach must rest on a firm base of supply market research spend analysis, customer requirements knowledge, supplier selection criteria, and other formal processes. Ultimately, integrating suppliers into a well-managed design phase is found to have a lasting effect on the competitiveness, long-term quality, and time it takes to get to market.
Conclusion
As the medical device industry faces uncertain times due to heightened global competition, healthcare reform, and a potential 2.3% federal tax on medical device companies’ total revenues, it now finds itself in the same crossroads as the automotive industry did in the 1990s. One thing is certain: Medical device companies must continue to develop and come to market with high-quality, innovative products while finding ways to drive costs from the device. Looking through the lenses of cost implications and production manufacturing during the early design phase will pay dividends down the road.
John Sapiente is the owner and president of Elgin Die Mold and Trident Manufacturing (Pinegree Grove, IL). Elgin Die Mold is a highly automated global supplier of safety critical components and subassemblies to the automotive industry with black box design, prototyping, testing, VAVE, manufacturing, and assembly. Trident Manufacturing is a medical device contract manufacturer providing new product development, design, prototyping, testing, full device manufacturing, Class 7 cleanroom assembly, sterilization service, packaging and kitting. He can be reached at [email protected] or 847/464-0140.
Related Content
Outsourcing Design and Manufacturing Services for Medical Devices Gains Momentum
About the Author(s)
You May Also Like


