September 8, 2015
Discover the elegant solution that Harvard University researchers came up with.
Chris Newmarker
|
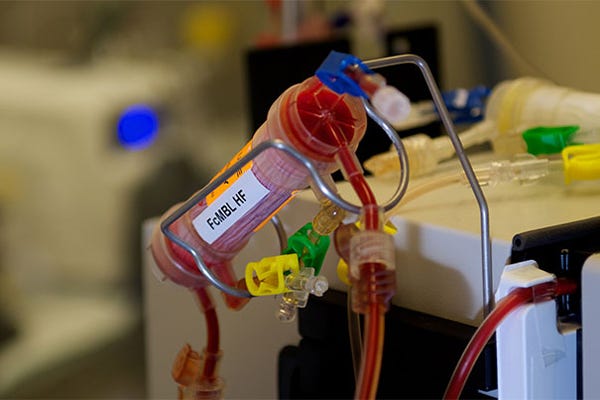
The blood-cleansing device is connected to a dialysis-like circuit . (Image courtesy of Harvard University's Wyss Institute) |
Researchers at Harvard University's Wyss Institute think they've hit on a strategy to get their unique blood-cleaning technology into clinical settings more quickly, and it was all about adopting as much existing medical technology as possible.
The research team originally had a prototype that worked like a dialysis machine, according to a Wyss Institute news release. Infected blood is flowed from one vein through catheters to the device, which has magnetic beads coated with a Wyss genetically-engineered blood protein called FcMBL. The FcMBL protein is inspired by a molecule already found in the in the innate immune system. It binds all types of live and dead infectious microbes such as bacteria, fungi, viruses, and the toxins they release.
Magnets in the original device then extracted both the FcMBL-coated beads and the pathogens stuck to them before blood was returned in another vein.
A major drawback for commercializing the device was that it was both complicated and novel--not a good situation when seeking regulatory approval, according to the Wyss Institute.
So what was the solution they came up with? They took the hollow fiber filters found in dialysis cartridges already approved by FDA, and then they coated the inner walls of the filters with FcMBL protein. In animal studies, the new device is able to eliminate almost all--more than 99%--of E. coli, Staphylococcus aureus, and endotoxins circulating in the bloodstream.
"If all goes well, physicians will someday be able to use the device in tandem with standard antibiotic treatments to deliver a one-two punch to pathogens, synergistically killing and cleansing all live and dead invaders from the bloodstream," Tohid Fatanat Didar, a postdoctoral fellow at the Wyss institute and a research fellow at Boston Children's Hospital, said in the Wyss news release.
The Wyss team is next moving to large animal studies.
"Since the development of earlier prototypes of the device, we've applied the Wyss model of de-risking the technology to prime it for commercialization," said Wyss Institute founding director Donald Ingber, leader of the Wyss team developing the device.
"Our goal is to see this move out of the lab and into hospitals as well as onto the battlefield, where it can save lives, within years rather than decades," Ingber said.
Learn more about cutting-edge medical devices at MD&M Philadelphia, October 7-8. |
Like what you're reading? Subscribe to our daily e-newsletter.
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like