October 25, 2013
It is probably one of the biggest medical device technology debates going on right now: Is Medtronic's MiniMed 530G, its first-generation artificial pancreas, really a game changer?
The answer is that the MiniMed 530G represents something between an incremental evolutionary step and a big leap forward.
|
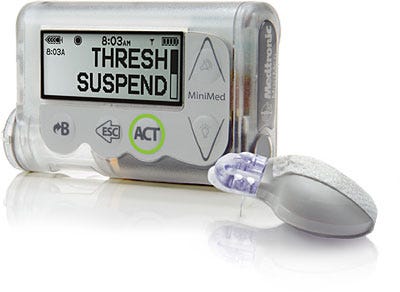
The MiniMed 530G can stop insulin delivery when sensor glucose levels reach a defined threshold. |
The smart insulin pump automatically stops insulin delivery when glucose levels in the blood fall below a preset level--not a small matter for diabetics worrying about a hypoglycemia-related seizure in their sleep.
Plus, Medtronic worked with San Francisco-based Bridge Design to create the Enlite glucose sensor used with the device. The new sensor has the advantage of not looking like a harpoon. Diabetics won't have to feel as though they're the title character in Moby Dick any more.
Phil Halbert, Bridge's director or engineering, says the "rounded shape, no harpoon-like form of the previous product, no exposed needle, its soft texture, and noise capture are all designed to reduce patient anxiety around insertion."
When it comes to glucose monitors, Medtronic is competing with San Diego-based Dexcom, which has been experiencing strong sales amid a patient-friendly reputation for its colorful, sleek-looking monitors.
"The real challenge for Medtronic's new pump is that [Dexcom] has a dramatically better CGM, and they don't work together," Ryan Winkler, a Minnesota state representative who has close relatives using such devices, said over Twitter.
While representing a step forward from earlier devices, the MiniMed 530G still does not reach the holy grail of a true "artificial pancreas" able to automatically adjust insulin delivery without a patient having to interact with the device.
Executives at Medtronic say it would be difficult to go from a completely open loop to a completely closed loop device in one step.
"We've taken the strategy from the very beginning that we're going to be working in a stepwise fashion with regulatory bodies. We think that makes the most sense from a patient safety perspective as well as being able to partner with regulatory bodies to get it approved," John Mastrototaro, vice president of research, technology, and business development, and chief technology officer at Medtronic Diabetes, tells MPMN's sister Medical Device and Diagnostic Industry publication.
Medtronic media relations recently shared Mastrototaro's answers to some MPMN questions about the technology and design goals behind the MiniMed 530G:
MPMN: How big of a leap forward is the MiniMed 530G over previous generation insulin pumps in your opinion (from a design and technology perspective)?
Mastrototaro: The MiniMed 530G with EnliteT is the first artificial pancreas device system approved by the FDA under its critical path initiative to accelerate the development and availability of a safe and effective artificial pancreas. The MiniMed 530G system includes Threshold Suspend automation, a feature that automatically stops insulin delivery when sensor glucose values reach a preset low threshold. This feature may help reduce the severity of hypoglycemia if the individual is unable to react and treat the event.
This is the first system with a feature that automatically intervenes in a particular case (when sensor glucose levels reach a preset low threshold and if the patient is unable to respond to the Threshold Suspend alarm), allowing people with diabetes to strive for better glucose control.
MPMN: What product development insights did you glean from working on this project?
Mastrototaro: It is important to understand how an individual would use the feature and how users need to be notified when the Threshold Suspend feature is activated.
MPMN: How did your team optimize the MiniMed 530G in terms of usability?
Mastrototaro is among the Medtronic Diabetes professionals featured in the above video. |
Mastrototaro: The Threshold Suspend automation feature shuts off insulin delivery for up to 2 hours when sensor glucose levels reach a preset low threshold value, if the patient is unable to react to the Threshold Suspend alarm. The threshold can be set from 60 to 90 mg/dL. The MiniMed 530G system also includes the new Enlite sensor, Medtronic's most accurate and comfortable glucose sensor available in the U.S. for continuous glucose monitoring (CGM).
The MiniMed 530G with Enlite shows a 31% improvement in accuracy over the previous generation (Paradigm Revel with Sof-Sensor). Additionally, the Enlite sensor is 69% smaller than the previous Medtronic sensor and it detects up to 93% of hypoglycemia episodes when predictive and threshold alerts are on.
MPMN: What were the biggest design challenges you encountered when developing the MiniMed 530G system and how did you overcome them?
Mastrototaro: Understanding the tradeoff between simplicity and functionality. We designed the feature so it could be easily understood, but still provide great utility. We also needed to assure the feature was safe even when system faults were present.
|
The Medtronic Enlite serter features a consumer-inspired design. |
MPMN: Can you summarize how your team was able to achieve a 31% improvement in accuracy of the continuous glucose sensor over the previous generation sensor?
Mastrototaro: Research demonstrated the value of reducing the size and trauma associated with inserting the sensor, so significant engineering focus was placed on addressing these issues. The Enlite sensor is less than 1/3 the size of the prior Sof-sensor.
MPMN: How would you like to see do artificial pancreas technology advance over the next five to 10 years?
Mastrototaro: At Medtronic our goal is to develop a fully automated artificial pancreas for people with diabetes--a system that would automatically deliver the amount of insulin you need with very minimal interaction from the patient. We are not there yet, even with the MiniMed 530G system.
However, the MiniMed 530G system is a critical first step on the journey toward that goal. With Threshold Suspend, it is the first FDA approved device that can stop insulin delivery temporarily if a sensor glucose value reaches a preset low level and the patient doesn't react to the alarm. This is the first system that takes action based on sensor glucose readings and the first product approved under the FDA's new category Artificial Pancreas Device System--Threshold Suspend.
This is one of three categories designed by the FDA as types of artificial pancreas device systems. The fact that the FDA has clearly defined these three categories is great news for the diabetes community, as it gives us a path forward in developing iterative steps toward a fully automated artificial pancreas system. Those iterative steps are important because a fully automated system is not going to arrive suddenly. Our plan is to step-by-step develop innovative technologies with increasing levels of automation in order to advance to our ultimate goal--a fully automated artificial pancreas system.
Qmed and MPMN editors Brian Buntz and Chris Newmarker contributed to this story.
About the Author(s)
You May Also Like