1990: The Safe Medical Devices Act of 1990 is signed into law, requiring hospitals and other facilities to report deaths, serious illnesses, and injuries associated with the use of medical devices.
July 8, 2014
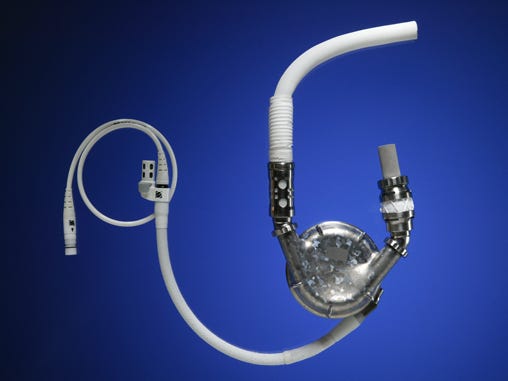
| 1991: The first patient to have a battery-powered left-ventricular assist device implanted leaves the hospital. Thermo Cardiosystems Inc. and the Texas Heart Institute developed and tested the device, and the patient is supported on the device for 505 days. |
1992: The Medical Device Amendments of 1992 are signed into law, establishing a single reporting standard for user facilities, manufacturers, and distributors, with respect to medical device reporting.
1993: CDRH reviews the quality of science in the review and approval of medical devices and recommends better-designed clinical trials and an increased use of randomized studies.
1997: The FDA Modernization Act of 1997 is signed into law, creating FDA’s Accredited Persons program to improve the efficiency and timeliness of the 510(k) process.
|
|
1980s | 2000s |
[image courtesy of THORATEC CORP.]
You May Also Like