In under 7 weeks, Sagentia developed a ventilator that minimized the need for standard components so it wouldn’t compete with existing needs. Hear the team’s story in a webcast.
June 18, 2020

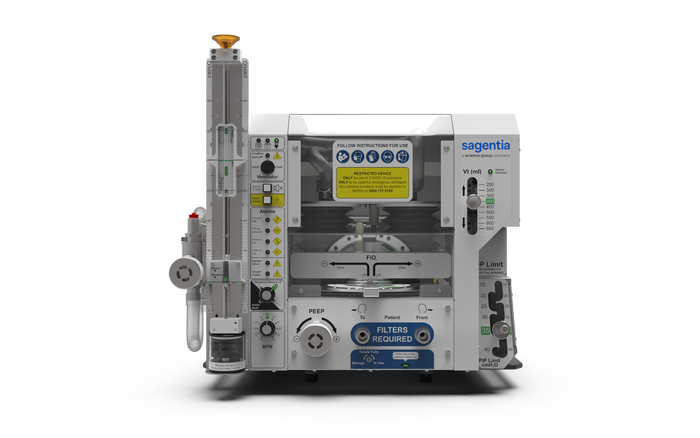
Early during the COVID-19 pandemic, health authorities were concerned about potential shortages of medical supplies and equipment such as ventilators. Several companies and innovators came to the rescue, and one remarkable response came from Sagentia Ltd., which answered the UK's Ventilator Challenge. Sagentia developed a ventilator in under 7 weeks.
“We responded with all our vigor and effort and the design that our team came up with was highly innovative and went after the brief unapologetically,” Dr. Rob Morgan, Vice President of Medical at Sagentia, told MD+DI. “The Sagentia Ventilator demonstrates the power of original thinking to solve unusual challenges.”
Morgan and Carl Hewett, Senior Product Development Consultant for Sagentia, explores the project during the upcoming webinar, The UK Ventilator Challenge: A Developer's Story, broadcast on June 25 and now available on demand. The event is part of MD+DI’s webinar series, How the Medtech Industry Can Respond to Crisis. Sagentia has posted a follow up article answering questions and sharing the results of a poll held during the webinar.
Morgan told MD+DI that the project entailed “designing a ventilator from scratch” and “minimizing the use of standard ventilator components so as not to compete with existing ventilator production.” The result was a design he described as “elegant in its simplicity and straightforward to fabricate.”
“We went for a largely mechanical design that avoided software completely to both fulfil the brief in avoiding conflict on part sourcing and simplify the development and approval process,” he explained. “We are proud that we achieved this in 7 weeks. We had a clinically viable design that was evaluated successfully at the government's designated test facility – generally the work of several years.”

The Sagentia team did encounter a few challenges, which Morgan and Hewett share during the webinar. “The development environment was highly challenging: there were extreme conflicts in the supply of components normally used in ventilator manufacture, employee social distancing was a necessity, and the clinicians needed in the design process were busy treating patients,” said Morgan. “But the crisis revealed new ways of collaboration between normally competing companies and with supply chain partners, and it allowed for an expedited regulatory pathway balancing the risk: benefit of rapidly developed devices. We learnt much that we will take forward into future product developments and we will share this learning in the webinar.”
They also share how the design process unfolded. “As an ISO13485 approved product designer, we designed the Sagentia Ventilator from the ground up for use in the critical environment of intensive care providing both mandatory ventilation and the support of spontaneous breathing, necessary to return a patient to breathing on their own,” said Morgan. “Throughout the process, it was carefully assessed against clinical need and under the guidance of the specialist team in the Technical Design Authority (TDA), which included NHS Clinicians and representatives from the Medicines and Healthcare products Regulatory Agency (MHRA) to determine its suitability to meet the UK’s immediate demand for safe and clinically effective mechanical ventilators.”
Please listen to the webinar, “The UK Ventilator Challenge: A Developer's Story,” moderated by MD+DI managing editor Omar Ford. The event is part of MD+DI’s webinar series, How the Medtech Industry Can Respond to Crisis.
Sagentia is a global science, product, and technology development company that partners with clients in the medical, consumer, industrial, and food and beverage markets. Sagentia employs more than 150 scientists, engineers, and market experts and is a Science Group company, which provides independent advisory and leading-edge product development services focused on science and technology initiatives. It has 10 offices globally, two UK-based dedicated R&D innovation centers, and more than 400 employees. Other Science Group companies include OTM Consulting, Oakland Innovation, Leatherhead Food Research, TSG Consulting, and Frontier Smart Technologies.
About the Author(s)
You May Also Like




