June 3, 2015
In this article, Insight Product Development walks through the steps needed to minimize risk in medical device product development while increasing your odds of creating a medtech masterpiece.
Craig Scherer
|
Craig Scherer is a senior partner and cofounder of Insight Product Development. |
Over more than 25 years, we've spent a substantial amount of time at Insight Product Development examining just how important it is to really understand device users, the environments devices are used in, and all the tasks that make up user workflows. We've also developed these devices with the rigor required of medical devices and with the ability to scale to production at the end of development.
Substantial technology considerations are required throughout the development process that can be the difference between success and failure. To turn a great concept into a medical device masterpiece requires a deep understanding of how to properly develop the technology components that maximize user functionality while minimizing development risks that an unproven set of technologies can bring.
Whether you are an early stage medtech startup or an already successful established medical device company, the same process applies. Making these steps central to your technology development process will help you mitigate risks across your development timeline and budget, as well as the device itself.
Hear Insight Product Development founder Craig Scherer speak on a panel titled "Design to Production: Turning a Great Design Concept into a Medical Device Masterpiece" on June 9 at MD&M East in New York City. |
Define What Technology Needs to Do
As part of every development program, the initial step is to establish user requirements and confirm them through testing. Once users have been studied and their needs documented, decisions around balancing these users' capability with the devices' level of complexity can be made. This allows the team to arrive at a set of requirements that will drive the selection of the appropriate enabling technology sets. The key here is to clearly understand and document what we need the device to do and then select and evaluate potential technologies that best meet these functional requirements based on our documented user capabilities.
Conduct a Technology Readiness Assessment
Once a number of technologies have been identified that can potentially satisfy our requirements, it is necessary to understand the readiness level of each technology relative to the individual application. The key at this stage is to characterize the core functionality of the technology to see how it behaves on its own. Sometimes spec sheets are all that is required for this but sometimes it is necessary to evaluate each technology on the bench to quantify performance characteristics.
Once the core functionality is characterized, the team can then evaluate the technology readiness across many variables to confirm it is not only appropriate functionally, but also is feasible to incorporate into our solution set. Readiness variables can include the cost acquisition, the cost to evolve or adapt, the ability to scale, the availability of production quantities, the ability to perform and function in multiple technology systems, among many other considerations.
It is important to remember that just because a technology is successfully used by a company within a single industry doesn't necessarily mean it will be appropriate in another.
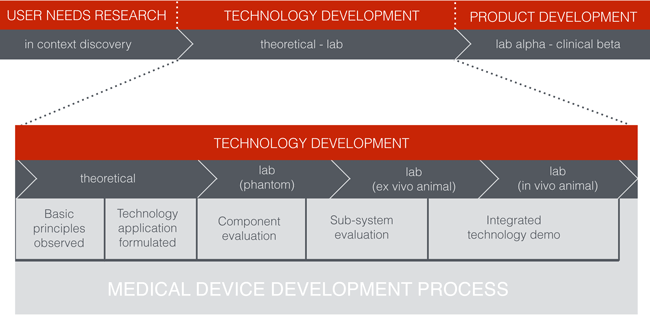
Build, Test, Repeat
Once the leading candidate technologies are selected, it is time to start evaluating them physically. The key to this part of the process is that the iterative cycle of building and testing should be working toward a higher order of confirmation at each step. Not only should the fidelity of the technology embodiment increase toward a more and more specific and refined solution, the environment and test parameters that it is evaluated in should also reach higher levels of fidelity.
It is important to avoid the urge to down-select too quickly. As the team moves forward, it is a wise approach to keep multiple technology options in the mix so that you don't limit your endgame should one option unexpectedly fail.
|
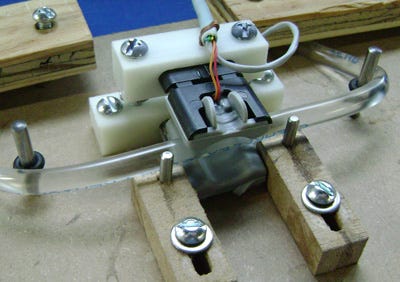
Component breadboard being tested on a phantom. |
Evaluate It on the Bench. Physical evaluation begins with examining the appropriateness of the physics or how it behaves in a single focused application. In this phase of analysis and development, the technology model is often tested on a representation of the physiological application sometimes called a phantom. Early testing units are often cannibalized from existing devices or improvised from scratch and tested on the bench for early confirmation of appropriateness of the individual technology component.
For example, Insight Accelerator Labs member, Briteseed, first tested their SafeSnips optical technology algorithm, which measures various physiological parameters of blood vessels, by using low cost, off-the-shelf hardware components and pulsing solutions through a phantom made from PVC tubing. This is typically when developers start to use tools like 3-D printing to rapidly develop breadboards to support these technology models. The outcomes from these efforts provide early confirmation of the technology application.
Develop Subsystems in a Simulated Environment. The next step is to begin developing individual subsystems of the device to evaluate performance of all of the components as an assembly. Like the technology, which began evolving into a higher level of fidelity, the test environment should as well. At this level, the subassembly should be able to fully function and perform a task in a simulated environment.
For example, a heating technology that is being developed for a device that will be used to ablate tissue would include the electrical system to power the actual technology, while a chicken breast simulating human tissue, might be used to test the ablation effectiveness.
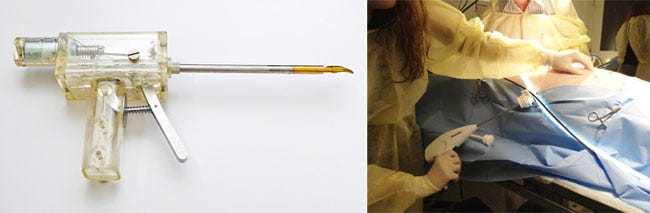
Put It All Together and Get Relevant. The final step in de-risking the chosen technology is to create an integrated technology demonstrator and test it in a relevant environment. A common environment is an animal lab, as it affords developers to a chance to test their fully integrated functional assemblies on live tissue to confirm technology core requirements at much higher fidelity. This integrated technology demonstrator should contain all subassemblies necessary to confirm effectiveness at the system level.
While integrated assemblies may not be pretty, they're built to test technical functionality--not for user evaluations, which will come later. The goals of this integrated technology demonstrator model are distinctly different than later stage integrated alpha prototypes engineered to prove appropriate functionality in even higher fidelity.
|
An integrated technology demonstrator model is on the left while an animal lab is shown on the right. |
Ready to Scale
Once all these activities are complete, all project requirements will have been established and you are ready to start scaling to your commercialized design. Making your device a viable commercial product can only begin once the technology package has been completely confirmed and de-risked. This stage is the traditional product development effort where the device becomes more relevant to the users. Within this stage, ergonomics are applied and tested, configurations are developed with higher volume manufacturing in mind, and technologies may even become invisible to the users.
It is critical throughout your technology development cycle to continuously reference your core user needs to ensure you resist the temptation to make the new device be all things to all people. Every additional technology added to the system at this stage not only has its own development risks but also adds significant risk to the larger program by adding complex interrelationships between technologies. This will most likely add significant cost, time and complexity to the development program. Focus instead on ensuring that your final commercialized system will meet all cost and timeline parameters and completely support clinicians' workflow and capability expectations.
Craig Scherer, co-founder and senior partner at Insight Product Development.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like