The ability of product designers to accurately predict changes in polymer properties is of critical importance to the medical device industry.
July 1, 1998
The introduction of new or modified products to the medical marketplace requires the assurance that they can be stored for an extended period (from one to five years) without any decrease in performance that may affect safety and efficacy when the products are used. Because full-period, ambient-aged samples usually do not exist for a such products, it is generally necessary to conduct accelerated-aging tests to provide experimental data in support of performance and shelf-life claims for these products until full-period samples become available.
The ability of product designers to accurately predict changes in polymer properties is of critical importance to the medical device industry. Modeling the kinetics of polymer deterioration is difficult and complex, and the difficulty is compounded by the fact that a single-rate expression of degradation or change developed over the short term may not be valid over the long-term service life of the product or material being studied. In order to design a test plan that accurately models the time-correlated degradation of medical polymers, it is necessary to possess an in-depth knowledge of the material composition and structure, end-product use, assembly and sterilization process effects, failure-mode mechanisms, and storage conditions.
 Accurate prediction of medical product shelf-life performance is critical. Photo: GATX Logistics, Inc.
Accurate prediction of medical product shelf-life performance is critical. Photo: GATX Logistics, Inc.
A given polymer may have many functional chemical groups organized in diverse ways (crystalline, glass, amorphous, etc.), along with additives such as antioxidants, inorganic fillers, plasticizers, colorants, and processing aids. It is the sum of these variations—combined with variations in product use and storage environment—that determines the degradation chemistry. Fortunately, the majority of medical products are constructed from a limited number of polymers that have been well-characterized over extended-use periods. A procedure known as the Simplified Protocol for Accelerated Aging (also called the "10-degree rule") was developed around the collision theory–based Arrhenius model. When applied to well-characterized polymer systems over moderate temperature ranges, the test results obtained can be within the required degree of accuracy.
The aging of products or materials refers to the variation of their properties over time, the properties of interest being those related to safety and efficacy. Accelerated product aging can be defined as a procedure that seeks to determine the response of a device or material under normal-usage conditions over a relatively long time, by subjecting the product for a much shorter time to stresses that are more severe or more frequently applied than normal environmental or operational stresses.
The primary reason for using accelerated-aging techniques in the qualification testing of a medical device is to bring the product to market at the earliest possible time. The goal is to benefit both the patient—for example, through early availability of a life-enhancing device—and the company—by generating additional sales and market share—without exposing either to any undue risk. Although accelerated-aging techniques are well documented in academic circles, information on the use of these techniques in medical product testing is somewhat limited. FDA has issued a handful of product guidances (for contact lenses, human drugs and biologics, etc.) that incorporate accelerated-aging methodology, but no official, broad-form agency policy currently exists. As a result, many medical product manufactures have developed custom accelerated-aging policies based on these guidances or other applicable referenced publications. (See the bibliography at the end of this article.)
Many accelerated-aging techniques used for the qualification testing of polymer medical devices are based on the assumption of zero-, first-, and pseudo-first-order chemical reactions following the Arrhenius reaction rate function. This function—long the basis for studying most chemical reactions—states that an increase or decrease in the reaction rate at which a chemical reaction proceeds changes according to the following equation:
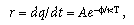
where r = the rate at which the reaction proceeds; A = the constant for the material (frequency factor);  = apparent activation energy (eV); k = Boltzmann's constant (0.8617 x 10–4 eV/K); and T = absolute temperature. With appropriate substitutions, the simplified expression for the 10-degree rule can be derived:
= apparent activation energy (eV); k = Boltzmann's constant (0.8617 x 10–4 eV/K); and T = absolute temperature. With appropriate substitutions, the simplified expression for the 10-degree rule can be derived:
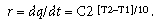
It should be noted that the 10-degree rule provides a conservative acceleration factor at room temperature for activation energies less than 0.7 eV. Because of the exponential effect, this can be conservative by orders of magnitude. (In some cases, a better match between the model and experimental room-temperature data can be achieved by modifying the 10-degree rule using an alternate temperature differential between 5° and 20°C that best fits the experimental data.)
The 10-degree rule will likely be conservative in the prediction of shelf life. However, the technique depends on numerous assumptions that must be verified by real-time validation testing conducted at room temperature for the targeted shelf-life. A well-designed product-release test program will involve the use of continued "room-temperature" aging that is always greater in age than the age of any product in use. This is especially important when using these techniques for the qualification of critical (life-saving) components or devices. The approach does involve some limited risk of potential recall, in the event that room-temperature-aged testing shows a significant deficiency following real-time-aged testing of the product. Applying accelerated-aging test techniques in conjunction with a comprehensive knowledge of the materials involved is a prudent method of doing business, with the benefits of early product introduction far outweighing the minimal risk of premature product failure.
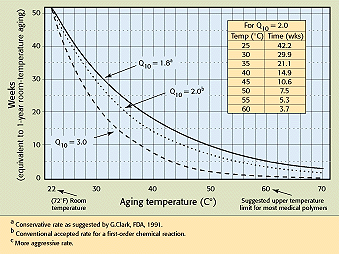
Figure 1. Accelerated aging of polymers (time versus temperature), showing the time (in weeks) equivalent to 1 year of room-temperature aging when a polymer is heat-aged at a selected temperature (°C). (Q10+ Δ 10°C reaction-rate constant, assuming a room temperature of 22°C.)
The functional properties of products depend on the properties of their constituent materials. Since these materials for the most part are polymeric in nature, their performance is related to the rate of degradation of their inherent structure and configuration over time. This degradation is primarily chemical—a combination of effects arising from oxidative chain scission, oxidation hydrolysis, changes in crystallinity, and other factors that may be environmentally dependent. Any stress factor that can reasonably affect the functional properties of the product over time should be included in the accelerated testing program. The testing is conducted at higher than usual levels of stress—whether of temperature, humidity, radiation, temperature cycling, chemical environment, or other factors. The results at these accelerated stress conditions are then correlated to those at normal operating or storage conditions using a physically appropriate statistical model such as the 10-degree rule.
A number of miscellaneous factors must be considered in conducting an accelerated-aging study, regardless of the accelerated-aging protocol employed:
When establishing the accelerated-aging protocol, the environmental conditions selected should not represent unrealistic failure conditions that would never occur under real-time, ambient-aged conditions. For example, where there is evidence that an aging effect occurs only in the presence of heat, one should perform aging under conditions of storage or use only.
Proof testing can serve as a substitute for destructive testing. Proof testing requires all samples to be tested at the end of each test interval to a specified failure point, then returned to the aging environment for continued exposure. This method, however, is only applicable when the selection of proof-test values does not weaken or compromise the product properties being examined.
When use of any accelerated-testing model produces a nonlinear plot, this may be an indication that multiple chemical reactions, complex reactions of a second or third order, or autocatalytic reactions are occurring at some, but not all, test temperatures. In these cases, elevated temperatures may negatively distort the performance of the material at operating or storage conditions. Under such circumstances, one should consider performing aging under conditions of storage or use (ambient).
When possible, accelerated testing should be employed—testing of the device or material at high stress for a short period of time in order to deduce the dominant failure mode. Based on knowledge of the principal degradation mechanisms and stresses on or within the part, a significant enhancement in test-plan efficiency can be achieved through the use of excessive environmental stresses such as heat, oxygen, chemicals, or radiation. Often, radiation is the best stressor to use, since the degradation pathways are often similar to those induced by heat or oxygen. Irradiating a product at 100 kGy (10 Mrd) or more before initiating a formal test program can often root out the product's "Achilles' heel" and allow for improved targeted test design. For products currently on the market, additional insight into the most probable modes of failure can be obtained from investigating customer complaint files.
All test units should consist of products constructed of the same components and subassemblies and manufactured by the same processes, methods, and procedures as those used for routine production. In addition, the product—in its final package—should be subjected to a minimum of one standard sterilization cycle. Additional sterilization cycles or combinations of different sterilization methods can be employed as needed to represent worst-case routine production.
For previously characterized polymers, a simplified approach for accelerated aging is based on conducting testing at a single accelerated temperature and then employing the rule stating that the rate of a chemical reaction will increase by a factor Q10 for every 10°C increase in temperature. The typical relationship selected for commonly used medical polymers is Q10 = 2—that is, a doubling of the reaction rate for each 10°C increase in the temperature above the use or storage temperature. The simplified protocol for accelerated shelf-life testing is not a replacement for a more complex and advanced accelerated-aging protocol, but is instead a protocol for systems known to conform to zero, first-order Arrhenius behavior. This type of conservative relationship is appropriate for a wide range of medical polymers that have been previously characterized.
Presented below is a list of the steps to be followed when designing an accelerated-aging test protocol using the simplified 10-degree-rule methodology:
1. Identify the ingredients in the polymer formulation of interest. Care should be taken to identify—both qualitatively and quantitatively—all additives (e.g., antioxidants), fillers, and processing agents. Combine this information with a thorough knowledge of the stresses on and within the part, identify the principal degradation mechanisms for the system, and select experimental techniques that accurately evaluate the degree of degradation and its impact on the performance of the product.
2. Select the reaction rate coefficient of Q10 = 2, unless another rate coefficient has been previously determined experimentally.
3. Select an ambient temperature representative of actual product storage and use conditions (normally between 20° and 25°C). A temperature of 22°C (72°F) is preferred for most disposable medical products, although any temperature that can be justified may be used.
4. In order to decrease the test time to the maximum extent, select a temperature for the accelerated-aging oven conditioning that is as high as possible, within the following limitations: (a) The accelerated-aging (oven) temperature must not exceed the material's glass-transition temperature (Tg), melt temperature (Tm), crystalline-forming temperature (Tα), or heat-distortion temperature less 10°C (THDT—10°C). This applies to all materials for which the above temperatures are greater than the ambient temperature. (b) The accelerated-aging temperature chosen should not be greater than 60°C, since the accuracy of the assumptions on which this method is based declines sharply as greater geometric multiplication factors are applied with greater deviation from ambient temperature. That is, any error will also be multiplied by the same factor, resulting in a greater and greater error effect. (c) The preferred test temperature of between 50° and 60°C should be selected unless particularly temperature-sensitive materials (such as PVC) are involved. At 60°C, the accelerated-aging time relationship is that 3.7 weeks is equivalent to 1 year ambient aging at 22°C (room temperature).
5. For any accelerated aging and ambient temperatures selected, the relationship of oven test time to shelf-life time is as follows:
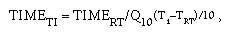
where T1 = oven aging temperature, TRT = room temperature (ambient/ use/storage), and Q10 = reaction-rate coefficient. As an example of the application of this formula, what test time in a 50°C oven would be required to achieve equivalency to 5 years of ambient shelf-life aging of a product at 22°C (i.e., T1 = 50°C, TRT = 22°C, Q10 = 2)? The correct response is as follows:
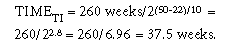
In other words, an oven test time of 37.5 weeks at 50°C would be equivalent to 5 years at 22°C ambient temperature (i.e., 7.5 weeks/year).
6. In cases where differences in coefficients of expansion in mating parts could contribute to significant stress generation and part failure, temperatures need to be cycled through high (accelerated aging) and then low temperatures. The high (accelerated aging) temperature is selected as described above in step 4, with the low (freezer) temperature < 5°C. During the low-temperature conditioning, no test time is accumulated toward the ultimate shelf-life equivalency of the product.
7. For certain polymer structures, long-term performance may be influenced by the effects of humidity extremes. If humidity is to be included in the test design, a relative humidity > 85% for high humidity conditions and < 20% for low-humidity conditions should be used.
8. Choose interim test intervals significant enough to detect the initial presence of failures, but not so frequent as to invoke undue hardship on resources.
9. Select the specific properties to be evaluated, and the tests to accurately evaluate any change in these properties.
10. Select a sufficient number of product test units so that statistically valid test results are obtained for each test interval. The test units should normally be finished product; however, subassemblies and even specially prepared test specimens are satisfactory in certain limited cases.
11. Test units drawn from the same product/material lot as the accelerated-aged units shall be ambient (real-time) aged and tested according to the same test regimen and at the same test intervals as the accelerated-aged test group. In addition, in order to control risk, ambient-aged product should be tested for the ultimate shelf life as well as for any interim test period selected.
12. Following the steps listed above, develop a written test protocol specifying the accelerated-aging conditions (temperature, humidity, heat cycling, time), time frames, sample sizes, and specific tests to be undertaken at each test time interval. Note that because of multiple test groups (accelerated-aged, ambient-aged, and control) and multiple test time intervals, sample sizes are relatively large and proper resource planning needs to be executed to ensure adequate accelerated aging oven space, ambient storage, personnel, and test equipment.
13. At the appropriate times, select samples from the aged and control groups and conduct testing as specified.
14. Evaluate the product test results using appropriate statistical methods, developing required statistical means, standard deviations, comparative t-tests, and F-tests to determine whether the product meets the design specification criteria or control group comparison for each test interval.
Use of the simplified protocol for accelerated aging can represent a valuable means for device manufacturers to obtain critical performance and shelf-life data on new products. There are, however, a number of issues that should be kept in mind by anyone using this technique.
Results will generally be conservative—that is, lesser shelf life will be obtained compared with that obtained via real-time aging. On the other hand, unrealistic negative outcomes may be produced because of heat degradation. It is important to remember that the protocol is based on the assumption that all materials in the study follow a zero- and first-order reaction-rate function, and that the supply of reactants remains constant over the study time frame.
The process works best when the chemistry of the degradation reactions is well understood, and when moderate aging temperatures are selected to minimize error factors and premature consumption of some reactants (e.g., antioxidants, radical groups) that may be sensitive to elevated temperatures. One must be alert for reaction-rate changes over test time frames; in such cases, use of multiple test temperatures or alternate accelerated-aging methodology is recommended. Use of a different methodology may also be advisable when a product is likely to include materials that have not been characterized previously. As with any accelerated-aging method, there is a degree of risk until the study is validated with real-time/ambient testing. Finally, the testing regimen should be designed to provide data that are appropriate to whatever criteria the product must ultimately satisfy.
A Review of Equipment Aging Theory and Technology, Electric Power Research Institute (EPRI) Report NP-1558, Philadelphia, Franklin Research Center, September 1980.
Clark GS, Shelf Life of Medical Devices, FDA (DSMA) report, April 1991.
Donohue J, and Apostolou S, "Shelf-Life Prediction for Radiation-Sterilized Plastic Devices," Med Dev Diag Indus, 12(1):124–129, 1990.
Gillen KT, and Clough RL, "Predictive Aging Results in Radiation Environments," Radiation & Physical Chemistry, 41(6): 803–815.
Gillen KT, Clough RL, and Wise J, Extrapolating Accelerated Thermal-Aging Results: A Critical Look at the Arrhenius Method, Albuquerque, NM, Sandia National Laboratories.
Meeker and Hahn, How to Plan an Accelerated Life Test—Some Practical Guidelines, vol 10, Milwaukee, WI, American Society for Quality Control, 1985.
Polymer Materials—Long-Term Property Evaluations, Underwriters Laboratories Report UP 764B, Northbrook, IL, Underwriters Laboratories, 1981.
Reich RR, Sharpe DC, and Anderson HD, "Accelerated Aging of Packaging: Consideration, Suggestions, and Use in Expiration Date Verification," Med Dev Diag Indus, 10(3):34–39, 1988.
Sandford C, and Woo L, "Shelf-Life Prediction of Radiation Sterilized Medical Devices," in Society of Plastics Engineers, Inc., Technical Papers, vol XXXIII (ANTEC 87), Brookfield, CT, SPE, 1987.
Shelton WS, and Bright DG, "Using the Arrhenius Equation and Rate Expressions to Predict the Long-Term Behavior of Geosynthetic Polymers," Geosynthetics '93 (Vancouver, Canada), Roseville, MN, North America Geosynthetics Society, 1993.
"Standard Practice for Heat Aging of Plastics without Load," ASTM Report D3045, West Conshohocken, PA, ASTM.
Woo L, and Cheung W, "Importance of Physical Aging on Medical Device Design," in Society of Plastics Engineers, Inc., Technical Papers, vol XXXIV (ANTEC 88), Brookfield, CT, SPE, 1988.
Karl J. Hemmerich is general manager and corporate technical advisor at Isomedix Corp.'s gamma irradiation facility located in Sandy, UT. He was formerly president of Ageless Processing Technologies, a consulting firm specializing in the medical disposables market, and has also worked at Ivac Corp., Cutter Laboratories, and Becton Dickinson. He was a member of the task force that developed the technical information report for postirradiation of materials (ISO 11137).
Copyright ©1998 MD+DI
About the Author(s)
You May Also Like


