In studying the field of 2015 Medical Design Excellence Awards finalists, we identified these noteworthy trends.
May 1, 2015

Jamie Hartford
Each year, the Medical Design Excellence Awards (MDEAs) call our attention to the most innovative medical devices on the market. But beyond simply highlighting noteworthy products, the program provides a peek into trends taking hold in the medtech industry. And because the jury includes clinicians as well as designers and engineers, the MDEAs also offer clues as to what product attributes are valued by the broader healthcare system.
In studying this year’s finalists, we identified these four trends.
Revolutionary Design
|
The Krabat Sheriff S2's unique saddle seat design enables more freedom of movement for users. |
Good design is always a cornerstone of MDEA finalists (it’s in the name of the competition, after all), but this year several products showed how the design of a device, rather than technological bells and whistles, can make a product revolutionary.
Consider the wheelchair, a medical device that has looked largely the same for decades. In developing a new version for children, Krabat AS threw the traditional wheelchair design, which features a flat seat similar to a regular chair, out the window. Instead, the Norway-based company’s Krabat Sheriff S2 features a saddle seat that separates users’ legs, opens the hips, and tilts the pelvis forward into a more neutral position to provide greater stability and freedom of movement. It can also prevent complications including muscle contractures, hip pain, and subluxations, according to the company.
“I have designed wheelchairs in my life and I have never seen anything like that before, so I was absolutely floored by the design,” says MDEA juror Pascal Malassigné, a career research scientist and industrial designer at the Milwaukee Veterans Affairs Medical Center.
The impact of great design was also evident in the user experience of many of this year’s finalist products.
“We have seen user interfaces that are so graphically illustrated that it was a pleasure to watch their operation and see how physicians, nurses, physical therapists, and technicians would be able to use it so easily and safely,” says juror Yadin David, founder of Houston-based Biomedical Engineering Consultants.
Products such as Maquet’s Servo-U and Servo-N ventilators made critical, lifesaving equipment safer and easier to use by innovating the user experience. Research by design firm Veryday identified a divide between users’ knowledge and the ventilator’s capacity, so the team focused on closing that gap with a touchscreen interface that includes features such as graphically illustrated settings previews, built-in tutorials, and automated calculations. The product also features three screen views: one for providers, one for distance viewing, and one with a soothing background that minimizes the information so as not to alarm or distract the patient’s visitors.
Finalists also showed that innovative design doesn’t have to be complicated. In fact, it shouldn’t be complicated at all.
The Renew Insert, made by Renew Medical Inc., is about as simple a device as you can imagine. It consists only of a silicone insert and a plastic fingertip applicator. But the product, which is intended to be placed inside the rectum to prevent fecal incontinence, won praise from the judges precisely for that simplicity of design.
“This device is simply an insertion valve that prevents this problem,” says juror Craig Jackson, program director for the Hartwell Foundation. “What was most notable about it was the clever design and ease of using the device by the affected individual. It’s a clever innovation addressing a market need that is neither sexy nor elegant but terribly, terribly useful.”
Consumerization Continues
Valedo uses wearable technology and gamification to guide users through back exercises. |
The consumerization of medical technology has been a trend for several years now, but this year’s MDEA finalists proved that it may finally be reaching critical mass.
“I think the digital environment is basically getting widespread and is finding itself into all kinds of categories in medical design, in interfaces for patient care and clinician-patient relationships,” Malassigné says.
One example of that is Merck Serono’s MSdialog, a cloud-based software platform to help patients with multiple sclerosis manage their disease with their doctors. Available as an Android and iOS app as well as through the Web, the program works with the RebiSmart drug-delivery device to log drug injections and capture information about the patient’s symptoms, as well as share that information remotely with healthcare providers.
“We’re getting spoiled by access to technology in many other areas, and we demand the same level of accessibility and quality also from our healthcare service,” says juror Marta Gaia Zanchi, founder and managing director of entrepreneurship consultancy Medinnovo.
As a result, medical technologies are merging with consumer devices, as seen in entries such as the ReSound LiNX, and Starkey Halo hearing aids, which stream sound from and can be controlled by users’ smartphones.
A number of this year’s finalists also translated trends from consumer tech into the healthcare realm. Hocoma's Valedo digital coach, for example, combined wearable technology with gamification to guide users through exercises to reduce back pain. Sensors monitor the user’s motion, which corresponds with the movements of characters in a digital game to help them perform the exercises correctly.
The Valedo device, which can be used at home and captures data to be shared with a physician or physical therapist, was also among a number of finalist products that are helping to continue the march of healthcare out of the clinical environment.
“Here in the U.S., a lot of focus on the Affordable Care Act and a lot of other policy changes has focused on outcomes and bundled payments that have physicians focusing more on the entire cycle of healthcare, not just the episodic care that occurs in the office periodically,” explains juror Stephanie Kreml, principal at healthcare consulting firm Popper and Co.
“Some of these devices will aid patients to take care of themselves at home, without intervention from a physician as frequently.”
In line with that trend were devices like the Sam Ultrasonic Diathermy Device, which stimulates tissue through acoustic energy to alleviate pain and speed healing of muscle and tendon injuries. This kind of therapy was traditionally delivered only in a clinical environment, but the wearable Sam device can be used at home, making it easier for patients to get the daily therapy that has been shown in clinical literature to be most effective.
Diagnostics Step Up
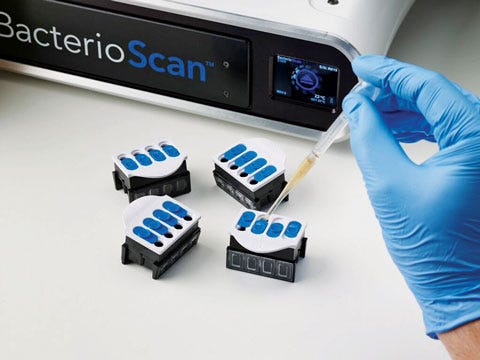
|
The BacterioScan Laser Microbial Growth Monitor can detect the presence of urinary tract infection in a sample in less than 90 minutes and detect sepsis in under three hours. |
One way healthcare systems in the United States and beyond can cut costs and improve care is by increasing the convenience, speed, and accuracy of diagnoses. And if this year’s MDEA finalists in the diagnostics category are any indication, manufacturers are churning out products that can make that happen.
“I found that the diagnostic device category was very strong this year,” says veteran MDEA juror Michael Wiklund, general manager of UL’s human factors engineering practice.
One product in the category, the Cologuard colon cancer screening test, offers a noninvasive, at-home alternative to a colonoscopy. After a patient receives a prescription for the test, a stool collection kit is sent to their home. Once the sample has been collected, the patient simply sends it back to the lab for analysis and waits to hear from their doctor with the results.
Another diagnostics finalist, the BacterioScan 216R Laser Microbial Growth Monitor, can speed detection of urinary tract infections and antibiotic resistance and susceptibility by more than 90%. This could reduce lab costs and labor, as well as decrease unnecessary use of antibiotics for treatment.
The True Margin is a tool to enable more accurate diagnosis of skin cancer from a smaller sample size. During Mohs surgery, tissue is methodically excised and examined for cancer cells on the spot until a clear margin is achieved. But a problem with this technique is that the current method of testing the tissue for cancer cells results in a high level of false-positive results, requiring more tissue to be removed and lengthening the procedure. In addition to reducing the sample size needed for testing, the True Margin reduces false positives by 13.6% and makes the procedure more efficient.
Greater Focus on Women’s Health
|
Coloplast's Speedicath Compact Eve urinary catheter was designed to resemble a woman's cosmetic product. |
Although women make up about half of the population, their unique healthcare needs have not always been given their due by the medtech industry. But judging by the number of women’s health products among this year’s finalists, that could be changing.
“There are several entrants grouped together in the women’s health category, which is interesting,” Kreml says. “In the past, most of the healthcare devices and medical devices have focused more on the generic male patient.”
What’s more, some of these devices went a step further than simply addressing women’s health issues. “We had quite a few in women’s health that have helped empower women,” Kreml says.
One such device is the Woman’s Condom developed by the nonprofit Program for Appropriate Technology in Health. Created as a woman-initiated method to protect against unintended pregnancy and sexually transmitted infections, the product uses a softer and thinner polyurethane sheath than other female condoms to provide good sensation for both partners.
Companies this year appeared to make a concerted effort to truly understand the needs of female users. Coloplast, for example, designed its SpeediCath Compact Eve hydrophilic-coated urinary catheter with a mindset of “the woman before the product.” Understanding that users may want to keep the function of the product discreet, the company housed the catheter in a compact package designed to look like a cosmetic product.
Join us in celebrating the Medical Design Excellence Awards Winners and Finalists at a special ceremony in conjunction with MD&M East in New York City, June 9–11, 2015. |
Jamie Hartford is MD+DI's editor-in-chief. Reach her at [email protected] or on Twitter @MedTechJamie.
You May Also Like