Designing and testing devices using 3-D physical simulation is a key part of developing a commercial product.
November 3, 2016
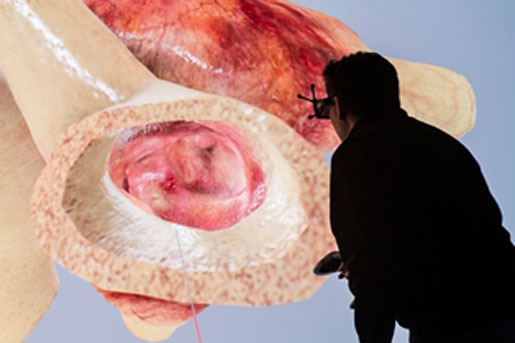
Designing and testing devices using 3-D physical simulation is a key part of developing a commercial product.
Steve Levine
The Living Heart Project enables realistic simulation.
At a time when the industry is facing some of its toughest challenges, more than 200 regulators, engineers, and healthcare leaders came together in Chicago at the American Medical Device (AMD) Summit in October, to discuss the state of the medical device industry and focus on opportunities to accelerate innovation, with increased predictability and profitability. From a regulatory, patient and payer perspective, medical device business models are changing, creating an environment that has rendered sustainable innovation elusive for many medical device companies seeking to grow their top line as well as bottom line.
These shifts in the marketplace are pressuring the balancing act between corporate efficiency, time to market, and predictable patient outcomes. It has raised an essential question for the future of the medical device industry--how to restructure to remain competitive and compliant while simultaneously meeting the needs of the patient, provider, and enterprise?
Learn about "The Crossroads of 3-D Printing Medical Devices and Legal Principles" at BIOMEDevice San Jose, December 7-8. |
The healthcare industry is finding answers in an unlikely place, by turning to a resource traditionally associated with more traditional manufacturing industries such as automotive and aerospace. Designing and testing devices "in-silico," or 3-D physical simulation, has become recognized as a core technology medical device makers need to enable regulatory science and accelerate the journey of bringing products to market.
What challenges are medical device makers faced with today?
With aging populations and rising quality of life expectations, global demand and competition for medical devices is driving unprecedented need for efficiency and cost management over the entire lifecycle of a product. This is in the face of increased data needs for regulatory approvals and value assessments for payer reimbursement. To flourish, medical device makers must change the way they innovate, design for manufacturability, and differentiate their products in the market. New models are needed to identify pathways for accelerated product development, improved quality, and patient safety, all while spending less and tracking more.
A looming business barrier faced by all device makers is compliance risk. These issues too often arise despite strides made to transform operations to digital. Many organizations, particularly small and medium size businesses, still rely on paper-based systems. As a result, data is stored in multiple siloed locations across the organization with no single unified change management system. To develop a high quality product, information must be capable of being shared among the engineering, quality and regulatory aspects of the device development process. To sustain the lifecycle of a product, information must continue to stream across the silos in order to keep devices current and applicable.
How can realistic simulation help medical device developers address these challenges?
Today, best-in-class manufacturers begin the product development process with design exploration in the digital world, unconstrained by the limitations of bench or animal testing. To do this effectively, realistic digital representations of the end-use environment is essential. In many industries, physics-based realistic simulation provides exactly this and then serves as a cost effective virtual test and design optimization environment throughout the product lifecycle. However, the physical conditions that replicate real world use of medical devices are often not well understood and simplistic bench testing environments are used instead. As a result, animals and ultimately humans become the proving ground of new designs, at unsustainably high cost and dramatically limiting the cycles for innovation due to increased life risk factors. More importantly, these physical tests often yield limited insight into underlying weakness in design, resulting in surprises in the market and too often, recalls. However, encouraging progress has been made in creating realistic virtual human environments, allowing device testing in realistic environments through the use of computational modeling and simulation.
Through 3-D design and simulation, medical device makers have access to a wide variety of models for human bone, muscle, blood, and other tissues, and for those who have made the digital transformation, they can collaborate around the same set of common data within their organization for more efficient design, testing, and evaluation of new ideas. The end result is that device makers can build and test products without having to create physical models, allowing for alteration and adjustment without the time and money spent on developing imprecise products, with all data organized and tracked automatically, accessible for regulatory submission.
How will realistic simulation decrease risk and drive evolution in healthcare?
Realistic simulation has massive potential to dramatically transform healthcare. 3-D technologies will change everything from patient education and physician training to enabling new innovations in devices and procedures and driving down the time and cost for FDA approvals. Demonstration of virtual efficacy will one day be a required surrogate for approval and may even be sufficient for derivative approvals. Even reimbursements will be accelerated through virtual patient data and virtual trials that demonstrate benefit well ahead of the time any human studies can be completed.
Looking only slightly further into the future, realistic simulation will play an unprecedented role in the emergence of personalized medicine. With the potential to precisely develop personalized therapies and unique treatments, simulation has the potential to offer cures and remedies to ailments never before thought possible. Already, 3-D printing is becoming standard practice for pre-surgical planning of congenital heart defect repair. Imagine that we will soon digitally animate these models to practice the surgery and be confident we can deliver the best outcome possible.
An example of how realistic simulation can be used to address these benefits is the Living Heart Project. In a previous MD+DI article, I explained how a simulated model of the human heart demonstrates the potential for computer-aided engineering (CAE) technology to revolutionize how medical devices are developed, tested, and regulated. Through CAE, simulation technology allows for truly individualized treatment and delivers greater insight into health and healthcare decisions. One year after the emergence of this technology, Living Heart is now available as part of an enterprise solution paving the way to a new generation of devices that are imagined, designed, and tested completely in the virtual world before ever touching the bench.
In addition to changing the pathway for development of medical devices themselves, 3-D modeling and simulation will also have an immense impact across the healthcare industry:
Developing combination products and associated pharmaceutical drugs
Accelerating clinical trials and approvals
Personalized medicine and pre-surgical planning
Insight into the human body reaction to various therapies
Physician education & training
Patient engagement from diagnosis to preventative care
As exemplified through the Living Heart Project, virtual human modeling and simulation has the opportunity to connect what hasn't been connected before in healthcare. By working with the same set of data, teams can work together and accumulate common knowledge to move innovation forward and provide superior patient health experiences. Even more importantly, simulation will help manufacturers connect with patients, providers who use their product in the field. These strengthened relationships are critical to accelerate product innovation, reduce development risks and costs, and still improve patient safety and product quality.
Steve Levine, PhD is senior director of product strategy and the executive director of the Living Heart Project at Dassault Systemes SIMULIA.
[Image courtesy of DASSAULT SYSTEMES SIMULIA]
You May Also Like


