February 25, 2015
|
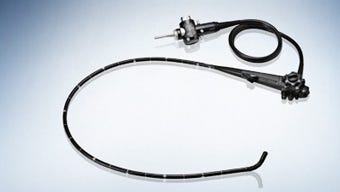
Olympus' TJF-Q180V duodenoscope, as shown on the company's website |
A lawsuit has already been filed against Olympus Corp. over its duodenoscopes, and FDA is facing pressure to do more.
Chris Newmarker
Medical device companies may want to pay even more attention to how easy it is to clean their reusable devices--not to mention the instructions they are issuing for cleaning--after a type of endoscope used in half a million U.S. procedures a year was linked to a dangerous "superbug" outbreak at UCLA.
An 18-year-old high school student hospitalized with a carbapenem-resistant Enterobacteriaceae (CRE) infection has sued Olympus Corp. in Los Angeles County Superior Court over its duodenoscope linked to the outbreak, according to the Los Angeles Times.
Aaron Young's lawsuit claims the Japanese electronics giant redesigned its Olympus TJF-Q180V duodenoscope last year, but provided hospitals and doctors with cleaning instructions for an older model. At least seven patients were infected and two died from the drug-resistant superbug at UCLA's Ronald Reagan Medical Center.
Meanwhile, FDA is facing criticism over not heeding reports over the years that ERCP endoscopes (also called duodenoscopes) were tough to clean and prone to spreading antibiotic-resistant bacteria among patients.
"This problem has been known since at least 1987," John Allen, MD, the president of the American Gastroenterological Association, tells CNN.
U.S. Rep. Ted Lieu, D-CA, has called for congressional hearings, noting that duodenoscope-linked outbreaks have also occurred in Pennsylvania, Illinois, North Carolina and Washington State in recent years. "Addressing the problems posed by duodenoscope-linked superbug outbreaks is one step forward in combating the health and national security threats posed by antibiotic-resistant bacteria," Lieu said in a press release.
Sold in the United States by manufacturers including Fujifilm, Olympus, and Pentax, duodenoscopes are threaded down through the digestive tract and into the small intestine. They provide the least invasive way of draining fluids from pancreatic and biliary ducts blocked by cancerous tumors, gallstones, or other conditions. But the duodenoscopes' movable "elevator" mechanism at the tip, while improving efficiency and effectiveness, are challenging to disinfect, according to FDA.
Olympus tells MD+DI in a statement that it is is making supplemental material, even an interactive checklist with video demonstrations, available, and Pentax also says it is providing more information.
FDA issued an official warning last week. The agency insists it has been working for years to strengthen cleaning and disinfection protocols of complex instruments like duodenoscopes, issuing a draft guidance in 2011 and accelerating work after the CDC notified the FDA of a potential association of multidrug resistant bacterial infections and duodenoscopes.
"We continue our work in collaboration with federal partners, health care facilities and manufacturers to evaluate alternative cleaning protocols, test antibiotic-resistant organisms to assess their susceptibility to high-level disinfectants and explore additional strategies to reduce the risk of infections, such as the use of surveillance cultures of duodenoscopes," William Maisel, MD, FDA's chief scientist for its Center for Devices and Radiological Health, said in a Monday blog post.
For now, the FDA is recommending that health providers closely follow manufacturing instructions for cleaning and processing, and discuss the risks with patients.
UCLA has taken the extra step of sterilizing duodenoscopes with ethylene-oxide gas. But Maisel told the LA Times that the FDA does not recommend routine sterilization using the toxic gas because it could leave residual levels harmful to patients, and could damage the scopes themselves.
Refresh your medical device industry knowledge at BIOMEDevice Boston, May 6-7, 2015. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like