January 14, 2016
Major duodenoscope maker Olympus and FDA failed to alert the U.S. public, even as hundreds became infected with superbugs, according to a U.S. Senate report released this week.
Chris Newmarker
|
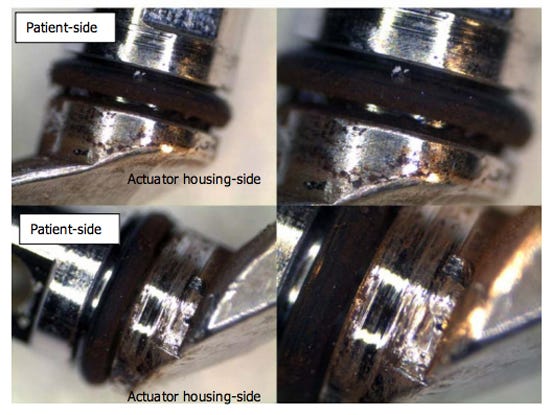
Pictures of a contaminated Olympus TJF-Q180V closed-channel duodenoscope, taken during a 2012 investigation in the Netherlands that Olympus participated in. The O-ring shows signs of wear, and the actuator-side area is heavily covered with brown scale. (Image from the Senate Health, Education, Labor, and Pensions Committee report) |
Potentially deadly superbug outbreaks caused by duodenoscopes were not isolated incidents. In fact, there were at least 25 outbreaks sickening at least 250 patients in the United States and Europe between 2012 and 2015, according to a report released this week by the top Democrat on the Senate Health, Education, Labor, and Pensions Committee.
Worse, it is likely that there are more incidents of infections linked to these devices that have never been identified, according to the report from the office of Sen. Patty Murray, D-WA. Reported outbreaks were primarily at large research hospitals and medical centers that are better at spotting antibiotic-resistant infections.
During much of the outbreak, major duodenoscope maker Olympus and FDA failed to alert the U.S. public, according to the report, which found faults in both FDA's 510(k) process and postmarket surveillance system. The report notes that Carbapenem-resistant Enterobacteriacea (CRE) kills almost half of those infected; Virginia Mason Medical Center in Seattle and UCLA's Ronald Reagan Medical Center are among the hospitals reporting deaths.
"The failure of FDA's device surveillance system to rapidly identify and respond to duodenoscope-related superbug and antibiotic-resistant infections serves as just one example of the fallacy of a system that is primarily reliant on hospitals and device manufacturers to self-report information to FDA," the report says.
The report recommends FDA evaluate whether repairs are needed to the duodenoscopes through a phased recall, and to clarify when manufacturers are required to to submit a 510(k) clearance notification to FDA. The report also calls for legislation to require and promote unique device identifiers (UDIs) in insurance claims, electronic health records, and device registries for faster FDA identification of problems.
Participate in a medical device companies regulatory boot camp at MD&M West, February 9-11 at the Anaheim Convention Center in Anaheim, CA. |
Sold in the United States by manufacturers including Olympus, Fujifilm, and Pentax, duodenoscopes are threaded down through the digestive tract and into the small intestine. They are used hundreds of thousands of times a year in the U.S., and provide the least invasive way of draining fluids from pancreatic and biliary ducts blocked by cancerous tumors, gallstones, or other conditions. But the duodenoscopes' movable "elevator" mechanism at the tip, while improving efficiency and effectiveness, is challenging to disinfect, according to FDA. The superbug outbreaks cropped up after the introduction of closed-channel duodenoscopes that were supposed to seal off the device's elevator wire channel from contaminants, but proved especially tough to clean and disinfect.
Olympus in a statement acknowledged that Democratic Senate HELP Committee staff made an important contribution toward understanding the factors behind what went wrong.
"Although we do not agree with all of the report's conclusions, we are closely reviewing the recommendations in the report as part of Olympus' ongoing efforts to increase patient safety associated with use of Olympus duodenoscopes," said Olympus spokesman Mark Miller.
Spokespeople for Fujifilm and Pentax told the LA Times that their companies will work with lawmakers and regulators to reduce infections.
FDA also appeared receptive. "We appreciate the report from Sen. Murray and will carefully consider its recommendations, many of which FDA is already taking steps to address. We agree with the senator that a broader approach to understanding how well duodenoscope devices work in real-time use is critical to public health," said FDA spokeswoman Deborah Kotz.
The Senate report backs up claims that an LA Times investigation made last month: Olympus for years failed to disclose the extent of problems to FDA and the U.S. public, even as it issued "important safety advice" in Europe in 2013, and another European safety alert about tainted scopes in 2014.
The report says: "The investigation found that by early 2013, Olympus, the manufacturer of 85% of the duodenoscopes used in the United States, knew of two independent lab reports finding that the closed-channel model duodenoscope could harbor and spread bacteria even after cleaning according to the manufacturer's instructions. Olympus never brought this information to FDA, and did not alert hospitals, physicians or patients in the U.S. to the risk of infection until February 2015."
None of the three duodenoscope manufacturers, and only one hospital with an outbreak, alerted CDC. The device makers and most hospitals also "largely failed to meet their legal obligations to provide complete and timely information about serious patient infections and deaths to manufacturers and/or FDA." And Custom Ultrasonics, a major manufacturer of devices to automatically reprocess the scopes, "failed at every level to meet basic expectations of transparency and openness and to actively engage with FDA to address contamination issues," the report says. (FDA ordered Custom Ultrasonics to recall the devices last year.)
As a result, FDA lacked critical information. Says the report: "Throughout 2014, FDA investigated the infections but did not issue any safety communications to inform hospitals of the risk posed by even duodenoscopes that are reprocessed according to manufacturers' instructions and reprocessed with cleared AERs."
It took FDA nearly 18 months from the time the agency learned of duodenoscope-linked infections for it to issue a safety communication, according to the report.
Problems with the 510(k) process also played a roll: "Two of the manufacturers [Olympus and Fujifilm] failed to seek FDA clearance before selling the 'closed-channel' duodenoscopes, all failed to adequately test whether the scopes could be cleaned reliably in real-world settings, and fully comply with adverse events reporting requirements."
It wasn't until early 2015 that enough news had been generated from the outbreaks to spark warnings from FDA, which divulged that Olympus' TJF-Q180V closed-channel duodenoscope had initially made it to market without a 510(k) clearance. By March, FDA was reporting that Olympus had "issued new, validated manual reprocessing instructions for the TJF-Q180V" as part of the review of a 510(k) application for the device, which FDA allowed Olympus to continue marketing while the application was under review.
FDA has issued warnings to Olympus, Fujifilm, and Pentax, ordering the three manufacturers to conduct postmarket surveillance studies, and elaborated on cleaning options for the scopes.
Here's the PDF of the full Senate committee report:
How Dangerous Scopes Needlessly Injured Hundreds
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like