The regulatory approval process for medical devices in Europe is certain to become more strict. Where are the new opportunities?
August 31, 2011
In a telling headline, Ben Hirschler (Reuters) illustrates the ongoing regulatory disintegration that is happening in Europe. The article "Heart Valves and Toasters: Call for New EU Rules," examines the CE mark process and questions whether the system is adequate.
It's a tension that all regulatory systems face. Europe enjoys earlier access to medical technology than many other countries.
In the United States, disgruntled medical technology firms look enviously at the relaxed regulatory regime in Europe and some Republicans have used the European example to scold the Food and Drug Administration over its slower approval process."
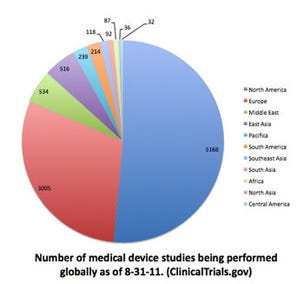 Increasingly, however, critics are asking the real price of such accessibility. Alan Fraser, a cardiologist at Cardiff University, led a review of the European Union regulations and presented his findings to the European Society of Cardiology (ESC).
Increasingly, however, critics are asking the real price of such accessibility. Alan Fraser, a cardiologist at Cardiff University, led a review of the European Union regulations and presented his findings to the European Society of Cardiology (ESC).
"It tends to be that clinical trials are done after approval in Europe but before approval in the United States," Fraser told Hirschler. "Where there have been isolated instances of devices that were associated with complications, those have disproportionately occurred in countries that have earlier approval—and that tends to be Europe."
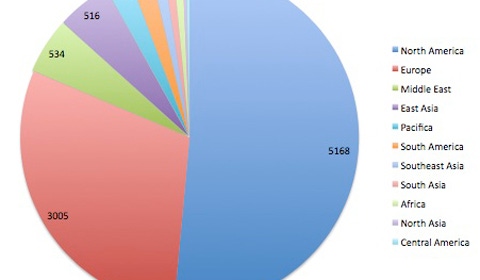
I have a feeling we will see Fraser's point of view gain traction, which will mean a change in how medical device manufacturers in the U.S. operate. If Europe adopts stricter regulatory processes (which is far more likely than the U.S. softening its process) clinical trials will have to change. It may be that Europe adopting stricter regulations will drive OEMs to go to Asia, the Middle East, and South America.
How would going first to these regions change the design of devices? Would there be more emphasis on portability, battery life, global use?
—Heather Thompson
You May Also Like


